NCEC-training-economic-evaluation
advertisement

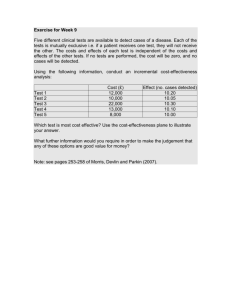
NCEC Economic Evaluation Training Shelley O’Neill Health Technology Assessment Directorate Presentation outline 1) Introduction to Health Technology Assessment (HTA) 2) NCEC guideline prioritisation criteria 3) Conducting a budget impact assessment 4) Reviewing economic literature Health Technology Assessment Objective: To inform safe and effective health policies that are patient focussed and achieve best value HTA is a “decision support tool” Health Technologies Includes a wide range of interventions used in healthcare and health promotion −Pharmaceuticals (Drugs) −Vaccines −Medical Devices −Diagnostics −Medical and surgical procedures −Public health activities Includes the systems within which health is protected and maintained Why do we need HTA? • Introduce technologies speedily with proven significant health • benefits Prevent the introduction of technologies which fail to meet the requirements of evidence-based analysis The best interests of the individual Fair & equitable allocation of resources for society Scarcity means that choices must be made ! Health Technology Assessment Science Patient wishes Industry claims Decision making “HTA is a decision support tool” Multidisciplinary process, summarises information about – Safety – Clinical and cost-effectiveness – Budget impact – Organisational impact / resource implications – Social and ethical issues related to use of a health technology in a systematic, transparent, unbiased and robust manner HTA for consumers/patients “rationing” HTA for Clinicians……. ..a clinical purist? ..or a financial realist? Economic Evaluation Cost effectiveness analysis: A form of economic evaluation which simultaneously assesses the costs and consequences (benefits) of an intervention Compare the costs and consequences (effects) of technology A vs technology B. HIQA: compare the costs per life year gained (LYG) or quality adjusted life years gained (QALYs) Economic Evaluation Questions addressed in an economic evaluation: 1. Can it work (efficacy)? 2. Does it work (effectiveness)? 3. Is it worth doing (efficiency)? 4. How much will it cost (affordability)? Outcome measure Life years gained: Number of years a patient’s/individual’s life is prolonged as a result of a particular intervention. Quality-adjusted life years (QALY): Number of years a patient’s/individual’s life is prolonged as a result of a particular intervention, incorporating adjustments for the quality of that life (morbidity) • Universally applicable to all patients and diseases • Allows comparisons between different health programmes • Useful for decision makers • QALYs allow for measurement of values or preferences for a particular health state Cost-effectiveness analysis Choice A Intervention • Compares costs and consequences of two technologies A and B Costs, BenefitsA Cost A – Cost B B Comparator / routine practice Effect of A – Effect of B Costs, BenefitsB • Incremental cost-effectiveness ratio (ICER) = Cost / QALY Economic evaluation Evaluation Type Costs Benefits Cost-effectiveness analysis (CEA) € Natural units (e.g. life years gained) Cost-utility analysis (CUA) € Health Status (e.g. QALYs or DALYs gained) Cost-benefit analysis (CBA) € € (e.g. based on willingness-to-pay) Cost-minimisation analysis (CMA) € Assume benefits to be equivalent Economic evaluations are usually in the form of CEA or CUA. Cost-effectiveness plane Cost (€) Q4 Intervention less effective and more costly Q1 Intervention more effective and more costly Effect (QALY) Intervention less effective and less costly Q3 Intervention more effective and less costly Q2 Cost-effectiveness plane The incremental cost-effectiveness ratio is usually represented graphically as a line passing through the origin on the cost effectiveness plane Cost (€) Q4 Q1 Accept (probably) €10,000/QALY Effect (QALY) Q3 Q2 Cost-effectiveness plane The incremental cost-effectiveness ratio is usually represented graphically as a line passing through the origin on the cost effectiveness plane Cost (€) Q4 €60,000/QALY Q1 Reject (probably) Effect (QALY) Q3 Q2 Cost-effectiveness plane The line passing through the origin represents our ‘acceptable’ cost-effectiveness ratio. That is our maximum (or threshold) willingness-to-pay for a unit of effect (life year or QALY). Cost (€) Q4 Q1 Effect (QALY) Q3 Q2 Willingness to pay Acceptability of a technology with an ICER value in the region of the threshold will be influenced by: • Degree of uncertainty in calculating the ICER • The innovative nature of the technology • Particular features of the condition and population receiving the technology • The wider societal costs and benefits NCEC Guideline Screening and Prioritisation Criteria Criteria 3 Economic Impact Would implementing this guideline have a substantial budget impact on the healthcare system ? Are there potential cost savings to be realised if the guideline is implemented? Is there national or international cost-effectiveness evidence to support implementing the guideline? NCEC Guideline Screening and Prioritisation Criteria Criteria 3 Economic Impact Would implementing this guideline have a substantial budget impact on the healthcare system? • Have the resource implications of implementing the guideline been considered? • Have the resources required for any initial set up or roll out phase been considered? • Have the cost of these resources to the publicly-funded system been estimated? Budget impact Would implementing this guideline have a substantial budget impact on the healthcare system? • Comprehensive economic evaluation tends to be both time and labour intensive • Budget impact analysis looks at the added financial impact of implementing a new clinical guideline over a finite period (this may be sufficient in most cases) • Typically only the direct costs to the publicly-funded health and social care system (HSE) in Ireland are included • Indirect costs (absenteeism from work, disability, need for long term care etc.) are important in instances where significant budget implications for other publicly-funded services or transfer payments are anticipated. Budget impact Budget impact matters – even when a technology is deemed cost effective ? Budget impact Assessing the budget impact can be broken into three distinct steps: 1. Identifying the resource use that may change 2. Estimating the size of these changes 3. Determining the relevant costs for these changes 1. Identifying the resource use that may change It is important to determine the treatment or intervention pathway: • How do the people receiving the intervention interact with the health services? • Who carries out the intervention and where? • How is onward treatment managed and monitored? 1. Identifying the resource use that may change • Are there any initial one-off resource requirements? (capital investment, staff training) • Will there be additional service utilisation arising from the intervention (Additional testing (e.g. biopsies), treatment, follow-up/monitoring, Primary/secondary/tertiary care) • Technology (Equipment purchase, ICT upgrading, test kits, Laboratory usage) • Harms/side-effects: will there be additional treatment of harms (medicines, surgical, alternative treatment pathway) Example from EWS What are the ongoing material costs? The early warning score chart is likely to replace currently used charts. These vary across sites with some consisting of a single sheet, however the change to the NEWS chart will have a negligible cost implication. Budget impact Assessing the budget impact can be broken into three distinct steps: 1. Identifying the resource use that may change 2. Estimating the size of these changes 3. Determining the relevant costs for these changes 2. Estimating the size of these changes • When evaluating the economic impact, it is the incremental impact that should be considered, the total cost of implementing the national guideline less what would have been spent on the current standard of care. • The comparator used should be ‘routine care,’ that is, the current or most widely used clinical practice in Ireland; in some cases this may be a mix of a number of different practices • Who will receive the intervention? How many patients/ individuals? • This could be presented as a range, to incorporate variation in the estimate or possible different scenarios. 2. Estimating the size of these changes Who will receive the intervention? The target population! The target population characteristics • age & sex • socio-economic status • life expectancy • co-morbidities • treatment response • etc will dictate how much of the intervention is required and will also impact on the treatment response. 2. Estimating the size of these changes Data we might be interested in and potential sources: • Morbidity oHospital In-Patient Enquiry Scheme (HIPE) www.hpo.ie oPrimary Care Reimbursement Service (PCRS) www.hse.ie/eng/staff/PCRS/ oNational Cancer Registry www.ncri.ie/ • Mortality oVital Statistics www.cso.ie oNational Paediatric Mortality Register www.sidsireland.ie/ oNational Drug-Related Deaths Index www.hrb.ie/ oNational Perinatal Reporting System (NPRS) www.hpo.ie 31 2. Estimating the size of these changes •Health service utilisation oHIPE & PCRS oBreastCheck www.breastcheck.ie & CervicalCheck www.cervicalcheck.ie/ oHealth Protection Surveillance Centre www.ndsc.ie/hpsc/ oNational Psychiatric Inpatient Reporting System www.hrb.ie/ • Demographic (Census) www.cso.ie • Exposure oNational Roads Authority www.nra.ie/ oOffice of Tobacco Control www.hse.ie/eng/about/Who/TobaccoControl •HIQA catalogue of health information sources: www.hiqa.ie/resource-centre/professionals/health-information-sources 32 Budget impact Assessing the budget impact can be broken into three distinct steps: 1. Identifying the resource use that may change 2. Estimating the size of these changes 3. Determining the relevant costs for these changes 3. Determining the relevant costs for these changes • Technology/intervention- (Data from the manufacturer) • Staff- include pay at mid-point of pay scale, employers’ PRSI, imputed pension cost, and overheads www.hse.ie/eng/staff/Benefits_Services/pay/ • Hospital in-patient and daycase procedure costs www.casemix.ie/ • Drugs covered on the community schemes (PCRS) www.hse.ie/eng/staff/PCRS/ • Hospital finance departments 3. Determining the relevant costs for these changes • Annual depreciation of any capital costs should be included in the analysis. • Include any maintenance contracts • If using cost from literature: o Retrospective health costs must be inflated to current prices using the Consumer Price Index (CPI) for Health. o Where costs are applied from other countries, all costs must be converted to euro using Purchasing Power Parity indices (PPP). o If transferring costs from another currency, the inflation should be calculated using the CPI for the local currency prior to conversion to euro using PPP. www.oecd.org/std/prices-ppp/ Staff training example from EWS What are the initial set up costs? Staff Training It was estimated that in total 20,500 staff will require training, training takes approx 8.5 hrs. The approximate cost for staff time spent training is €7.3million. A ‘train the trainer’ model was used for training with approx 300 staff delivering training, at 8hrs per sessions and each trainer delivering 6 or 7 sessions. The staff time cost involved to deliver training is an estimated €172,000. These are opportunity costs, that is diverting staff members from their usual activities to attend and provide training, rather than an actual cash cost to the HSE. NCEC Guideline Screening and Prioritisation Criteria Criteria 3 Economic Impact Are there potential cost savings to be realised if the guideline is implemented? • Are there any potential cost savings due to changes in the use of resources? • Have the benefits from improved outcomes been quantified and the associated costs or savings been estimated? Cost savings Are there potential cost savings to be realised if the guideline is implemented? • Savings from treatment avoided due to improved health outcomes (e.g. fewer bed days), a reduction in adverse events or stopping or changing current practice (different material costs). • Particularly relevant for preventive care – use of intervention may result in reduced health service use • Although introduction of a new guideline may lead to a reduction in staff requirements, it may be difficult to realise any potential savings (e.g., redeployment of staff). Cost savings example from NEWS What are the cost savings from improved outcomes? Introducing a NEWS will improve patient outcomes by reducing the number of unplanned admissions to ICU and reducing the number of cardiac-respiratory arrests. ICU additional cost = €1,316 per day extra general acute ward. Additional days on ward = 5 A saving of €6580 per patient not admitted to ICU. In 2011, approx 3,750 inpatients were diagnosed with a cardiac or respiratory arrest, where this was not the main reason for their admittance to hospital. Assuming a 26% reduction in those hospitals currently without a EWS (based on literature) there could be €4.2million or 3,200 ICU bed days saved per year. NCEC Guideline Screening and Prioritisation Criteria Criteria 3 Economic Impact Is there national or international cost-effectiveness evidence to support implementing the guideline? • Is a summary of the cost-effectiveness evidence presented? Is this generalisable or relevant to the Irish healthcare setting? • Has this evidence been gathered using systematic searching methods and are these methods documented? Review of economic literature Is there national or international cost-effectiveness evidence to support implementing the guideline? • Detailed guidance provided in the recently published draft guidelines for the retrieval and interpretation of economic evaluations of health technologies • Currently out for public consultation http://www.hiqa.ie/getting-involved/consultations/HTAguidelines Review of economic literature Is there national or international cost-effectiveness evidence to support implementing the guideline? • Has intervention/drug been assessed by HIQA or NCPE? • Systematic, transparent, unbiased and robust, evidencebased review is needed • Similar to clinical effectiveness/guideline review • Same methods for defining search question (PICOs) and reporting search strategy (inclusion/exclusion criteria, flow chart etc.) Search for economic evidence • Systematic literature search to identify cost effectiveness evidence should be reproducible, thorough and transparent, consistent with the search for clinical effectiveness/guidelines • Specialised databases (DARE, NHS EED, HTA Database www.crd.york.ac.uk/CRDWeb, www.thecochranelibrary.com). • Search should be performed using the major health search engines such as MEDLINE and EMBASE using economic search filters (available www.york.ac.uk/inst/crd/intertasc/econ.htm) Identifying relevant studies • • • Is it current? older studies may include outdated practices How applicable is the study to the Irish context? Are the populations, interventions and healthcare system similar to the Irish setting? Welte ‘knockout criteria’ o The relevant technology is not comparable to the one that shall be used in the decision country o The comparator is not comparable to the one that is relevant to the decision country o The study does not possess an acceptable quality Appraising the evidence Appraising the evidence: • Is the study of adequate quality? • Are there major limitations in the study design or in the economic modelling? Available tools in considering the quality of economic studies (CHEC-list, BMJ, Philips) Assessing Relevance and transferability ISPOR 1. 2. 3. 4. 5. 6. 7. 8. Relevance (population, interventions, outcomes, context) Credibility (external, internal & face validity) Model Design (type, assumptions, structure) Data (values & how they were obtained) Analysis (uncertainty) Reporting (sufficient detail) Interpretation (fair and balanced) Conflict of interest (steps taken) Synthesising and summarising the results • Can any inconsistencies in the results be explained? (e.g. different settings, perspectives used, prevalence rates, input costs) • What are the possible inferences that may be drawn for the likely cost-effectiveness of the intervention in the Irish setting? • Summarise limitations and applicability of the studies Practical Exercise From reading the selected Appendix XV for the Prevention and Control MRSA – A National Clinical Guideline Consider if each of the prioritisation criteria are met? •Would implementing this guideline have a substantial budget impact on the healthcare system ? •Are there potential cost savings to be realised if the guideline is implemented? •Is there national or international cost-effectiveness evidence to support implementing the guideline? Appraisal of Clinical Guidelines Relevant criteria 10. Systematic methods have been used to search for evidence on effectiveness and cost-effectiveness to ensure that the clinical guideline is based on best available evidence. The full search strategy should be clearly outlined 14. The health benefits, side effects, risks, cost-effectiveness, resource implications and health service delivery issues have been considered in formulating the recommendations 23. The potential budget impact and resource implications (equipment, staff, training etc.) of applying the recommendations have been considered Guidelines Guidelines for the Budget Impact Analysis of Health Technologies in Ireland 2010 www.hiqa.ie Useful websites Ireland –HIQA HTAs/Guidelines: www.hiqa.ie –National Centre for Pharmacoeconomics: www.ncpe.ie –NCEC Guideline Developers Manual: http://www.patientsafetyfirst.ie/images/stories/docs/ncec_ guidedevmanual_v2.pdf International Collaborations and Organisations –http://www.eunethta.eu/ –http://www.inahta.org/ –http://www.htai.org/ –www.euroscan.org.uk Databases –http://www.crd.york.ac.uk/crdweb/ –http://www.hta.ac.uk/ –http://www.inahta.org/HTA/Database/ HTA explained –http://www.nlm.nih.gov/nichsr/hta101/hta101.pdf Further Reading Silvia Evers et al. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. International Journal of Technology Assessment in Health Care, 21:2(2005), 240–245 J Caro et al. Questionnaire to Assess Relevance and Credibility of Modeling Studies for Informing Health Care Decision Making: An ISPORAMCP-NPC Good Practice Task Force Report. Value in health 17 (2014) 174-182 Z Philips et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technology Assessment 2004; Vol. 8: No. 36 (Appendix 3 Quality assessment in decision-analytic models: a suggested checklist) Robert Welte et al. A Decision Chart for Assessing and Improving the Transferability of Economic Evaluation Results Between Countries. Pharmacoeconomics 2004; 22 (913) 857876. Ron Goeree et al. Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. ClinicoEconomics and Outcomes Research 2011:3:89-104 MF Drummond et al. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996; 313:276-83 Thank You