sp 3 hybridisering
advertisement

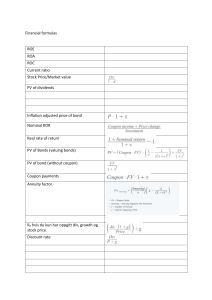
sp3 hybridisering http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch02/ch2-3-1.html C har 2 elektroner i skal-1(dem glemmer vi nu) og 4 elektroner i skal-2(disse 4 er vigtige for kemiske bindinger). I skal-2 er der plads til 4 par elektroner, et par i kugleformet S-orbital(’elektron-ballon’), 1 par i 3 p-orbitaler i x,y,z retningen (formet som langstrakt ballon, klemt sammen på midten, de står vinkelrette på hinanden. En orbital=’elektron-ballon’, elektron sky, der viser hvor elektronerne er det meste af tiden "Blend" (en blanding/hybrid, man taler derfor om hybridisering) the s and the three p orbitals... o since we "mixed" 4 orbitals, we get a set of 4 sp3 orbitals o each sp3 hybrid contains a single unpaired electron The sp3 hybrid orbital looks like a "distorted" p orbital with unequal lobes. The 4 sp3 hybrids point towards the corners of a tetrahedron(pyramide, de peger længst muligt væk fra hinanden!). Farvede er ‘positive’, hvide dele er negative, farvede dele lagt sammen bliver større ballon; farvet og hvid trækker fra hinanden, mindsker ballonen, derfor er farvede del større end den hvide. You can view an animation of the hybridisation of the C orbitals if you wish. For methane: o 4 equivalent C-H σ bonds can be made by the interactions of Csp3 with a H1s Now ethane, H3C-CH3 as a model for alkanes in general: o Both C are sp3 hybridised. o 6 C-H σ bonds are made by the interaction of C sp3 with H1s orbitals (see the red arrows) o 1 C-C σ bond is made by the interaction of C sp3 with another C sp3 orbital (see the green arrow) De to ender, CH3 , CH3 enhederne kan frit rotere omkring C-C-aksen/linjen Summary sp3occurs when a C has 4 attached groups the 4 sp3 hybrids point towards the corners of a tetrahedron at 109.5o to each other each sp3hybrid is involved in a σ bond sp2 hybridisering http://www.mhhe.com/physsci/chemistry/carey5e/Ch02/ch2-3-2.html "Blend" ( hybrid mellem) the s-‘kuglen’ and the 2 p orbitals... o since we "mixed" 3 orbitals, we get a set of 3 sp2 orbitals o the other p orbital remains unaffected o each sp2 hybrid and the p orbital contains a single unpaired electron In ethene, H2C=CH2 o both C are sp2 hybridised. o 4 C-H bonds are made by the interaction of C sp2 with H1s orbitals (see red arrows) o 1 C-C bond is made by the interaction of C sp2 with another C sp2 orbital (see green arrow) o 1 C-C bond is made by the interaction of the C p with the other C p orbital (see black arrows)- De 6 atomer ligger i samme plan. bond fås når de blå og røde dele smelter sammen, det giver ’elektron-ballon’ som pølser over og under planen(de brune pølser på figuren herunder. Denne pølse forhindrer at de to CH2 , CH2 enheder kan rotere omkring CC -aksen/linjen) Summary sp2 occurs when a C has 3 attached groups the 3 sp2 hybrids point towards the corners of a triangle at 120o to each other(orange vinkel) each sp2 hybrid is involved in a bond the remaining p orbital forms the bond view a double bond as a + bond sp hybridisering Blend" (i.e. hybridise) the s and a p orbital... o since we "mixed" 2 orbitals, we get a set of 2 sp orbitals o the other two p orbital remain unaffected o both sp hybrids and the 2 p orbitals contain a single unpaired electron (note the sp hybrids are offset horizontally to allow both lobes to be seen) In ethyne, HCCH o both C are sp hybridised o 2 C-H bonds are made by the interaction of C sp with H1s orbitals (see red arrows) o 1 C-C bond is made by the interaction of C sp with another C sp orbital (see green arrow) o 2 C-C bonds are made by the interaction of the 2 pairs of C p orbitals (see black arrows) Summary sp occurs when a C has 2 attached groups the 2 sp hybrids point in opposite directions at 180o to each other each sp hybrid is involved in a bond the remaining p orbitals forms the 2 bonds view a triple bond as a + 2 bonds Now is a good time to try a few questions about bonding and hybridization. orbitaler=elektron skyer, viser hvor elektronerne er det meste af tiden