Identification - Khazar University
advertisement
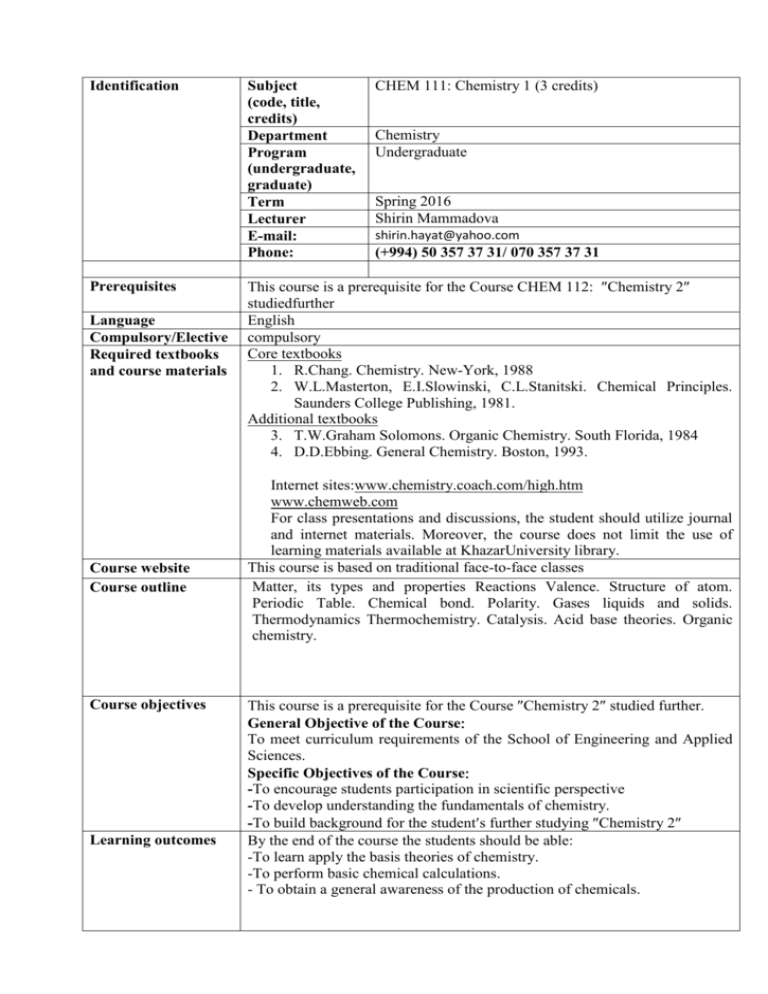
Identification Prerequisites Language Compulsory/Elective Required textbooks and course materials Course website Course outline Course objectives Learning outcomes Subject (code, title, credits) Department Program (undergraduate, graduate) Term Lecturer E-mail: Phone: CHEM 111: Chemistry 1 (3 credits) Chemistry Undergraduate Spring 2016 Shirin Mammadova shirin.hayat@yahoo.com (+994) 50 357 37 31/ 070 357 37 31 This course is a prerequisite for the Course CHEM 112: Chemistry 2 studiedfurther English compulsory Core textbooks 1. R.Chang. Chemistry. New-York, 1988 2. W.L.Masterton, E.I.Slowinski, C.L.Stanitski. Chemical Principles. Saunders College Publishing, 1981. Additional textbooks 3. T.W.Graham Solomons. Organic Chemistry. South Florida, 1984 4. D.D.Ebbing. General Chemistry. Boston, 1993. Internet siteswww.chemistry.coach.com/high.htm www.chemweb.com For class presentations and discussions, the student should utilize journal and internet materials. Moreover, the course does not limit the use of learning materials available at KhazarUniversity library. This course is based on traditional face-to-face classes Matter, its types and properties Reactions Valence. Structure of atom. Periodic Table. Chemical bond. Polarity. Gases liquids and solids. Thermodynamics Thermochemistry. Catalysis. Acid base theories. Organic chemistry. This course is a prerequisite for the Course Chemistry 2 studied further. General Objective of the Course To meet curriculum requirements of the School of Engineering and Applied Sciences. Specific Objectives of the Course -To encourage students participation in scientific perspective -To develop understanding the fundamentals of chemistry. -To build background for the students further studying Chemistry 2 By the end of the course the students should be able -To learn apply the basis theories of chemistry. -To perform basic chemical calculations. - To obtain a general awareness of the production of chemicals. Teaching methods Evaluation Policy x Lecture x Group discussion Experimential exercise Case analysis Simulation x Course paper Others Methods Date/deadlines Percentage (%) Aprel 2016 30 Midterm Exam 20 Assignment and quizzes 10 Presentation/Group Discussion May 2016 40 Final Exam 100 Total Attendance The students are required to attend all classes as a part of their studies and those having legitimate reasons for absence (illness, family bereavement, etc.) are required to inform the instructor. Tardiness / other disruptions. If a student is late to the class for more than 10 (ten) minutes, (s)he is not allowed to enter and disturb the class. However, this student is able to enter the second double hours without delaying. Exams In order to be excused from the exam, the student must contact the dean and the instructor before the exam. Excuse will not be granted for social activities such as trips, cruises and sporting events (unless you are participating). The exams will all be cumulative. Most of the questions on each exam will be taken from the chapters covered since the last exam. But some will come from the earlier chapters. In general the coverage will reflect the amount of the time spend in class on the different chapters. Withdrawal (pass / fail) This course strictly follows grading policy of the School of Engineering and Applied Sciences. Thus, a student is normally expected to achieve a mark of at least 60% to pass. In this case of failure, he/she will be referred or required to repeat the course the following term or year. Cheating / plagiarism Cheating or other plagiarism during midterm and final examinations will lead to paper cancellation. In case, the student will automatically get 0 (zero), without any considerations. Professional behaviour guidelines The student shall behave in the way to create favorable academic and professional environment during the class hours. Unauthorized discussions and unethical behavior are strictly prohibited. Use of any electronic devices is prohibited in the classroom. All devices should be turned off before entering class. This is a university policy and violators will be reprimanded accordingly For successful completion of the course, the students shall take an active part during the class time, raising questions and involving others to discussions. Learning and Teaching Methods This course considers active learning process rather than passive one. Wee k 1 2 3 4 Date/Day (tentative) Feb 12 2016 Feb 15−20 2016 Feb 22−27 2016 Feb - March 29−5 2016 Tentative Schedule Topics Topic 1Tools of Chemistry (1-32) Chemistry today (2), Science and its methods (3), Some basic definitions(5), Chemical Elements and Periodic table (9) Topic 1Tools of Chemistry (1-32) Measurement(10), Units of measurement(11), Handling number(17), The factor-label method of solving problems(22) Topic 2 Atoms, Molecules and Ions (33−82) The atomic theory (34), The structure of the atom (35), Mass relationships of atoms(40) Topic 2 Atoms, Molecules and Ions (33−82) Molecules: atoms in combination(47), Ions and Ionic Compounds (51), Percent Composition by Mass of compounds(53), Laws of Chemical combination (59), Experimental determination of atomic and molecular masses(61), Naming inorganic compounds (63) Topic 3: Stoichiometry: The arithmetic of Chemistry (83−134) The chemical equations (84) Writing chemical equations Balancing chemical equations Topic 3: Stoichiometry: The arithmetic of Chemistry (83−134) Properties of aqueous solutions(90) Electrolytes versus nonelectrolytes Types of Chemical Reactions (92) Combination reactions Decomposition reactions Displacement reactions Hydrogen displacement Metal displacement Halogen displacement Metathesis reactions Neutralization reactions Textbook/Assignments [1] [1] [1] March 7-12 2016 5 6 7 March 7-12 2016 March 14-19 2016 March - Aprel 28-2 2016 Topic 3: Stoichiometry: The arithmetic of Chemistry (83−134) Amounts of Reactants and Products(102) Limiting reagents(107) Yields: theoretical, actual, and percent(110) Concentration and dilution of solutions(113) Concentration Dilution of solutions Gravimetric analysis(118) Acid−Base titrations (120) Topic 4: Thermochemistry (135−166) Some definitions(136) Energy changes in Chemical Reactions(138) Enthalpy (139) Topic 4: Thermochemistry (135−166) Calorimetry (144) Hess’s law(149) Standard enthalpies of formation and reaction(154) Heats of solution and dilution (157) Topic 5: The gaseous state (167−216) The three states of matter (168) Substance that exist as gases (169) Pressure of a gas (170) The Gas laws(173) The ideal gas equation (181) Topic 5: The gaseous state (167−216) Stoichiometry involving gases (189) Dalton’s law of partial pressures (192) The kinetic molecular theory of gases (198) Graham’s law of diffusion and effusion (203) Deviation from ideal behavior (207) Topic 6: Chemical Bonding : Basic concepts (305−342) Lewis dot symbols (306) Elements that form ionic compounds(306) Lattice energy of ionic compounds(309) Topic 6: Chemical Bonding : Basic concepts (305−342) The covalent bond(314) Electronegativity(316) The octet rule(320) [1] [1] [1] [1] 8 Aprel 4-9 2016 Formal charge and Lewis structure(324) The concept of resonance(327) Exceptions to the Octet rule(329) Strength of the covalent bond(333) Topics 1 -2- 3 - 4 - 5 - 6 [1] Midterm exam 9 Aprel 11-16 2016 Aprel 18-23 2016 Topic 7: Chemical Bonding: Molecular geometry and molecular orbitals (343−394) Molecular geometry(344) The valence shell electron pair repulsion (VSEPR) model(346) Dipole moments(357) Valence Bond theory(361) Hybrodisation of atomic orbitals(363) Hybrodisation inn molecules containing double and triple bonds (373) Molecular Orbital theory (376) Molecular Orbital configurations(380) Delocalised Molecular orbitals(387) Topic 8: Intermolecular forces and liquids and solids (395 – 450) The kinetic molecular theory of liquids and solids(396) Intermolecular forces(396) The liquid state(403) Crystal structure(408) X−ray diffraction of crystals(417) Types of crystals(419) Amorphous solids(424) Phase changes(427) Phase diagrams(439) [1] [1] 11 Aprel 25-30 2016 Topic 9: Physical properties of solutions (451−492) Types of solutions (452) A molecular view of the solution process (452) Solutions of liquids in liquids (454) Solutions of solids in liquids (455) Concentration units (458) Effects of temperature on solubility (465) Effects of pressure on the solubility of gases (468) Colligative properties of electrolyte solutions (469) Colligative properties of nonelectrolytes (481) [1] 12 May 2−7 2016 Topic 10 : Oxidation – Reduction reactions (493−522) Oxidation – Reduction reactions: definitions (494) Oxidation numbers (495) Types of Redox reactions (499) Balancing Redox reactions (504) Quantitative aspects of redox reactions(510) [1] 13 May 9−14 2016 Topic 11: Organic chemistry Hydrocarbons (950) Alkanes, alkenes, alkynes, aromatic hydrocarbons Functional groups (965) [1] 14 May 16−21 2016 15 May 23−28 2016 May 2016 Alcohols, Ethers, Aldehydes and Ketones, Carboxylic acids, Esters, Amines, Chemistry in action : Petroleum industry (972) Review of the topic (950-972) Tutorials Tutorials [1] [1] Final exam May 2016 This syllabus is a guide for the course and any modifications to it will be announced in advance.



