Aspartame - Margaret Roberts
advertisement
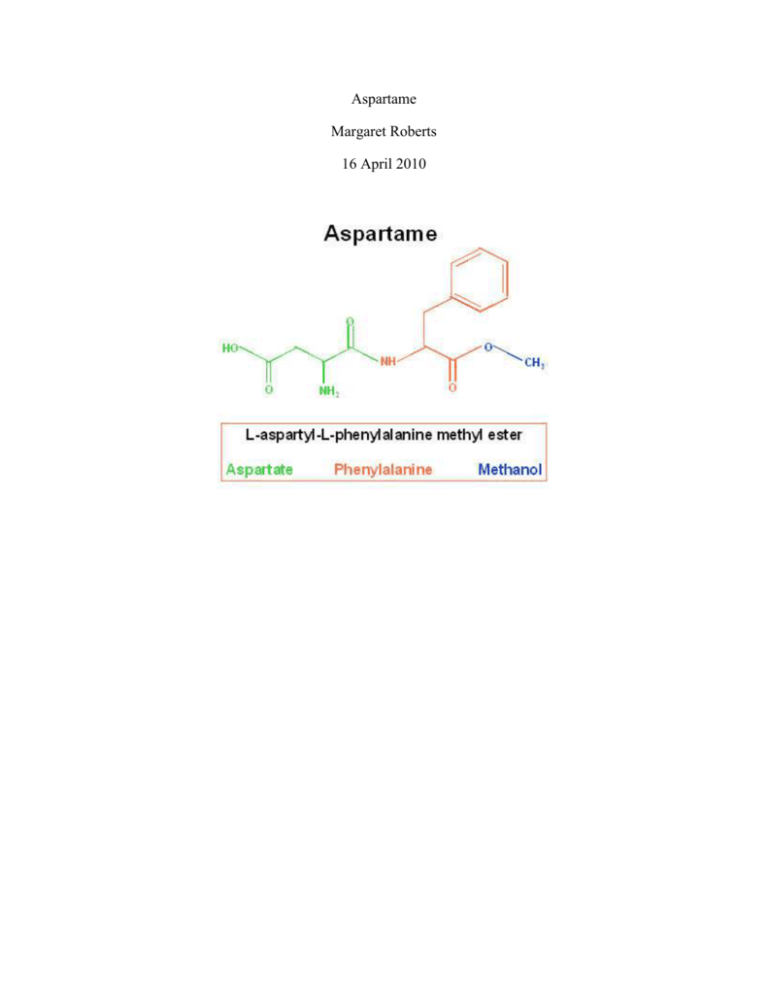
Aspartame Margaret Roberts 16 April 2010 Equal, NutraSweet, and aspartame are some of the more popular names for the compound N-(L-α-Aspartyl)-L-phenylalanine 1-methyl ester. It is a non-saccharide sweetener that is used as a sugar substitute in many foods. The molecular formula of aspartame is C14H18N2O5, or HO2CCH2CH(NH2)CONHCH(CH2C6H5)COOCH3, which combines for a molecular weight of 294.3 grams/mole (Chemical Land). Aspartame is a dipeptide, meaning that it is composed of two amino acids joined by the linking between one amino acid remnants and the carboxyl group of the next amino acid by hydrolysis into linear, branched or cyclical structures. The two amino acids of aspartame are aspartic acid and phenylalanine (Calorie Control Council). Aspartame is a white, odorless powder that is very sweet to the taste. Its sweetness is said to be 180-200 times sweeter than sucrose (Ajinomoto Food Ingredients). Its melting point is around 250oC and its density is 1.347 g/cm3 (Chemical land). Like other peptides, Aspartame breaks down into its amino acid substituent under high temperatures and high pH, so it is undesirable for baking (Ajinomoto Food Ingredients). This compound is composed of a carboxylic acid group, an amine group, Amide group, Ester group and an aromatic ring (Figure 1). Figure 1: N-(L-α-Aspartyl)-L-phenylalanine,1-methyl ester This compound was founded in 1965 when James Schlatter was working with amino acids to try to find a cure for ulcers. Luckily, Mr. Schlatter did not use very safe lab techniques, because he licked his finger to attempt to pick up a piece of paper and tasted a sweet flavor (Ajinomoto Food Ingredients). However, once he founded it, it had to be approved by the United States Food and Drug Administration. Aspartame was rumored to cause many adverse side effects, including brain tumors and other types of cancer. In 1981, it was approved for dry goods. In 1983, it was approved for use in carbonated beverages, other beverages, and baked goods. Finally, in 1996 most of the restrictions on aspartame were uplifted. However there are some stipulations to the approval. The Aspartame Information Service writes, “The revised regulation resulting from this approval eliminates the itemization of categories, as well as a weight restriction on aspartame's sale in bulk tabletop sweetener form. The only limitation that remains is a maximum 0.5% aspartame content in baked goods, which still allows aspartame to be used as the sole sweetener in any baking application” (Ajinomoto Food Ingredients). The compound has been approved for safety a total of twenty-six times (Ajinomoto Food Ingredients). As recently as 2006, the safety has been called into question. A one-million dollar study found that at high rates of consumption, for example, a 150-pound person drinking four or five bottles of diet soda a day, in lab rats can cause high rates of lymphoma and leukemia (Werner). The FDA, however, does not see a reason to change the laws (Werner). In addition, the Calorie Control Council claims the research is “not valid because the rats used in it had been allowed to live longer than the two-year standard established by the United States government's National Toxicology Program” (Werner). Most of the studies are biased, however, because the studies in support of aspartame are paid for by its supporters, while the studies against aspartame are paid for by its disparagers. Aspartic acid and phenylalanine are also found naturally in protein containing foods, including meats, grains and dairy products. Aspartame is digested like a protein, not like a saccharine. Upon digestion, aspartame breaks down into three components (aspartic acid, phenylalanine and methanol), which are then absorbed into the blood and used in normal body processes. Neither aspartame nor its components accumulates in the body. These components are used in the body in the same ways as when they are derived from common foods. (Calorie Control Council). However, one problem with aspartame is the substituent amino acid phenylalanine. One in ten thousand people suffer from a genetic disorder called phenylketonuria. The metabolic system is unable to digest phenylalanine, which causes a lethal accumulation of the amino acid. The Food Standards Agency says, “Sufferers …need to follow a very strict diet in order to limit their intake of phenylalanine, which is a normal constituent of proteins in food. Since aspartame is also a source of phenylalanine, all food products containing aspartame are clearly labeled to indicate the presence of phenylalanine so that those people who suffer from phenylketonuria can avoid consuming these products. This labeling is a legal requirement (Food Standards Agency). Aside from this disorder, many consumers choose aspartame over sugar because it tastes like sugar, however it lacks the calories of sugar. It is also believed to intensify some flavors, like fruit. It is also beneficial for diabetics because it allows them to eat sweet foods without interfering with their blood glucose levels. In addition, aspartame does not cause tooth decay like sugar does (Ajinomoto Food Ingredients). After ingesting aspartame, protein receptors on the taste buds detect the sweet flavor. There is only one receptor for sweet flavors and it is formed by the proteins T1R2 and T1R3 (Vaaman). Amazingly, both sugar and artificial sweeteners are able to bind to them. While our taste buds can be fooled, our brain is not tricked so easily. When fMRI scans were performed upon athletes drinking either sugar and artificial sweeteners or just artificial sweeteners, there was an unconscious response independent of the sweetness reception. The combination of artificial sweetener and sugar activated two reward-association areas in the brain- the striatum and anterior cingulated. The artificial sugar rinse alone did not activate either brain areas (Vaaman). Upon digestion, aspartame is metabolized into three substituents, methanol, phenylalanine, and aspartic acid (Roberts). Phenylalanine has two stereoisomers, Lphenylalanine and D-phenylalanine. L-phenylalanine is an essential amino acid, meaning it is required in animals but it cannot be synthesized in the body. L-phenylalanine gets converted into L-tyrosine which is further converted into L-DOPA, which is the precursor to neurotransmitters norpinephrine, epinephrine, and dopamine. Phenylalanine uses the same active transport channel as tryptophan to cross the blood brain barrier. In large quantities, it hinders with the synthesis of serotonin, another neurotransmitter (Wikipedia). D-phenylalanine does not contribute to the synthesis of proteins, and the biological functions of it are unknown. Phenylalanine can be degraded into two fragments, both of which can enter the citric acid cycle. Four of Phenylalanine’s nine carbon atoms yield free acetoacetate, which can be converted into acetoacetyl-CoA, then Acetyl-CoA. The second four carbon atoms are recovered as fumarate. The final carbon is lost as CO2 (Nelson and Cox 696). Aspartic acid is not an essential amino acid; however it is the precursor to four essential amino acids: methionine, threonine, isoleucine, and lysine. Like phenylalanine, aspartic acid enters the citric acid cycle as oxoacetate after it is converted in a catabolic pathway (Nelson and Cox 702). Methanol is broken down into formic acid and formaldehyde, which is damaging to many tissues in the body (Nelson and Cox 202). Even though it is one of the most tested products currently on the market, the debate about the safety of aspartame still rages on. Currently, the Food and Drug Administration has administered as a safe product as long as it is not eaten in too large of amounts, or more than fifty milligrams a day, which would be equivalent to eighteen or nineteen cans of diet soda a day (Mayo Clinic Staff). Aspartame is broken down into completely natural products that are found in everyday food; however caution should still be used if it is consumed every day. Works Cited Ajinomoto Food Ingredients LLC. The Aspartame Information American Service. 7 April 2010 <http://www.aspartame.net/index.asp>. Calorie Control Council. Aspartame Information Center. 2010. 7 April 2010 <http://www.aspartame.org/aspartame_facts.html>. Chemical Land. 2008. 7 April 2010 <http://chemicalland21.com/lifescience/foco/ASPARTAME.htm>. Food Standards Agency. Aspartame. 17 June 2008. 7 April 2010 <http://www.food.gov.uk/safereating/chemsafe/additivesbranch/sweeteners/55174#h_4>. Google Images. <www.images.google.com>. Mayo Clinic. Artificial sweeteners: A safe alternative to sugar? 8 April 2010. 14 April 2010 <http://www.mayoclinic.com/health/artificial-sweeteners/MY00073>. Nelson, David L. and Michael M. Cox. Principles of Biochemistry. New York: W.H. Freeman and Company, 2008. Roberts, H.J. Texas Heart Institute Journal. 2004. 12 April 2010 <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC387446/?tool=pmcentrez>. Vaaman. Sugar-free Satisfaction: Finding the Brain's Sweet Spot. 29 December 2009. April 14 2010 <http://www.voicetamil.com/?p=15561>. Werner, Melanie. "The Lowdown on Sweet?" The New York Times 12 February 2006: <http://www.nytimes.com/2006/02/12/business/yourmoney/12sweet.html?ei=5090&en=f 5f173a4cc33d534&ex=1297400400&partner=rssuserland&emc=rss&pagewanted=all>. Wikipedia. <www.wikipedia.com>.