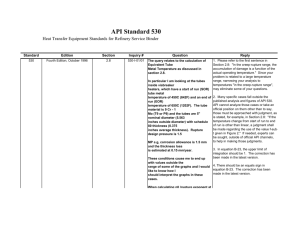
Physicochemical properties of APIs and their relevance to formulation
advertisement

Pharmaceutical Development Training Workshop on Pharmaceutical Development with focus on Paediatric Formulations Protea Hotel Victoria Junction, Waterfront Cape Town, South Africa Date: 16 to 20 April 2007 | Slide 1 of 39 April 2007 Physicochemical Properties of APIs and their relevance to formulation Presenter: Peter York Professor of Physical Pharmaceutics Institute of Pharmaceutical Innovation (IPI), University of Bradford, UK (www.ipi.ac.uk) (p.york@bradford.ac.uk) | Slide 2 of 39 April 2007 Physicochemical Properties of APIs and their relevance to formulation Outline of presentation Assurance of quality and safety of APIs Spectrum of tests and criteria for specifications for APIs Inter-dependency between ‘categories’ of properties ‘Functionality’ testing related to formulation design Summary of ‘challenges’ in API procurement and in evaluating APIs for formulation design | Slide 3 of 39 April 2007 Sources of APIs - Procurement Patented compounds – from originators or their licenced suppliers Non-patented APIs and generic APIs - transition from traditional supplying countries to other emerging nations eg India, China - consistency between ‘tender’ samples and following supplies from chosen supplier - pressure regarding CoG issues; no compromise with APIs for quality and safety | Slide 4 of 39 April 2007 Specifications and Standards for APIs API suppliers – for patented compounds Likely to be ‘licenced’ manufacturing agreement with originator, according to originator’s documentation ‘Drug Master File’ (submitted to registration authorities) containing full details regarding synthesis, testing and analytical procedures, impurities (sources and limits), storage requirements; drug source will be originator or their licenced toll manufacturer ‘Certificate of Analysis’ provided by API supplier; details results of routine tests applied to specified batches | Slide 5 of 39 April 2007 Specifications and Standards for APIs Specific pharmacopoeial monographs for off-patent/generic APIs Pharmacopoeias (eg BP) initiate new monographs for APIs approaching end of patent life, with support/dialogue with originator companies USP, EP, Int Ph, and national pharmacopoeia (eg JP, BP) Additional general guidance chapters and information provided in pharmacopoeias (eg testing methods…..) | Slide 6 of 39 April 2007 Generalised Content of API Monographs Objective – to provide the standards required to ensure the quality and safety of API compounds; appropriate limitation of potential impurities rather than provide against all possible impurities Monographs generally focus on chemical identification and purity assessment Chemical properties – - structure, molecular weight and chemical formula, melting point, moisture content - identification tests - solubility in common solvents - impurities, related substances (resulting from synthesis, and potential of degredants from storage during shelf life of API), and limits for their contents - assay | Slide 7 of 39 April 2007 Generalised Content of API Monographs Increasing awareness of need to monitor physical, crystallographic and ‘functional’ properties – some testing required by pharmacopoeial monographs Such information can provide valuable aid to formulation design Physical properties - moisture content - solid state/crystallography (eg polymorphism, level of solvation, crystalline/amorphous character) - particle properties (eg particle size) Storage recommendations NB indication of availability of reference standards provided | Slide 8 of 39 April 2007 Pharmacopoeial Monographs for APIs Nomenclature/structure - follow international agreed systems (eg rINN, BAN; CASRN)) Identification – means of verifying identity of API is as stated on the label - often two tests detailed –(for EP first is used in all circumstances, second if API complies with all other aspects of monograph) Impurities/related substances - specific, discriminating analytic methods - substances controlled related to synthetic route (eg reagents, catalysts) - limits imposed by monographs (and general guidelines) - additional limits for known degredation products if API unstable on storage | Slide 9 of 39 April 2007 Pharmacopoeial Monographs for APIs Assay - often a precise, non-specific (eg volumetric assay) test detailed - can use alternative assay method if known that alternative method will give a result of equivalent accuracy - purity figure related to reference substance - local reference material can be used if calibrated against official reference material - limits (range) based on data obtained in normal analytical practice, taking into account normal analytical errors, and acceptance of some variation in material eg aspirin – 99.5 – 101.5% (EP) eg erythromycin – sum of the contents of erythromycin A, B and C- 93.0 to 102%, with erythromycin B - maximum 5%, erythromycin C - maximum 5% (EP) | Slide 10 of 39 April 2007 Aspirin Molecule - Structure | Slide 11 of 39 April 2007 Erythromycin Molecules- Structure | Slide 12 of 39 April 2007 Pharmacopoeial Monographs for APIs Storage recommendations - to avoid/minimise degradation for sensitive materials - to avoid/minimise any contamination - possible vectors leading to degradation - elevated temperatures, light, oxygen (free radicals), moisture/high humidity, microorganisms eg aspirin – store in an air tight container (EP) eg erythromycin- protect from light (EP) | Slide 13 of 39 April 2007 API Routine Testing – ‘Good Practice’ Provide assurance of quality and safety Verification of CoA and magnitude of testing programme Sampling programme/isolated quarantine storage areas Retention/storage of batch samples Training programmes for staff, SOPs, GLP and validation of methods ‘Confidence’ in consistent quality of supply from chosen suppliers | Slide 14 of 39 April 2007 API Properties – Formulation Design and Processing 50% of new APIs, and many others, have very low aqueous solubility which can constrain drug dissolution (ie rate of solution) and thereby limit bioavailability Many APIs exhibit polymorphism (also solvation – hydration) – alternative molecular packing of the same chemical in crystalline material leading to different properties such as dissolution rate) Moisture content control – hygroscopic material often difficult to process (eg tabletting); change in hydration state (eg during wet granulation) Respiratory drug delivery – DPIs and suspension MDIs require drug particle size (aerodynamic) of 1 – 5 microns All above are also examples of QUALITY issues when formulating and processing APIs; may require additional testing and/or control procedures | Slide 15 of 39 April 2007 API Properties – Formulation Design and Processing Additional tests (sometimes specified in monograph, or testing methods detailed in pharmacopoeias) Examples – - solubility/dissolution (ie rate of solution) - polymorphism (eg IR analysis) - chirality (pure chiral API compared with racemate – HPLC with chiral colomn, capillary electrophoresis (CE)) - particle sizing (eg microscopy, sieves) or particle surface area (eg gas adsorption, permeability) - particle sizing for inhalation products (eg cascade impactors for aerodynamic particle size measurement | Slide 16 of 39 April 2007 API Properties - Solubility Descriptive solubilities General rules – – Polar solutes dissolve in polar solvents – Non-polar solutes dissolve in non-polar solvents | Slide 17 of 39 April 2007 API Properties - Solubility Many drugs are weak acids or weak bases Dissociation (ionisation) constants and pea Change in degree of ionisation and relative solubility of weakly acidic and weakly basic drugs as a function of pH Formulation and drug delivery issues pKa of aspirin (weak acid) = 3.5 | Slide 18 of 39 April 2007 API Properties - Polymorphism Representation of two polymorphic forms of a crystal consisting of a molecule represented by a ‘hockey-stick’ shape e.g carbamazepine, ritonavir | Slide 19 of 39 April 2007 API Properties – Crystallinity The disruption of a crystal (represented as a brick wall), giving the possibility for water vapour absorption in the amorphous region | Slide 20 of 39 April 2007 API Properties – Crystallinity API pretreatment effects on crystallinity The amorphous content of a model drug substance following milling in a ball mill and a micronizer (Ahmed et al 1996). | Slide 21 of 39 April 2007 API Properties – Formulation Design and Processing Alternative pre-treatment and processing of APIs (eg alternative final solvent used during final crystallisation step during synthesis of API; use of crystallisation rather than milling process for particle size reduction ) can lead to different surface properties of particles, such as interparticle cohesion and surface ‘charge’ These phenomena can lead to different secondary processing behaviour and potentially variation in product performance | Slide 22 of 39 April 2007 API Properties – Particle Size Analysis Microscopy – equivalent diameters Different equivalent diameters constructed around the same particle. | Slide 23 of 39 April 2007 API Properties – Particle Size Analysis - eye-piece graticule: circles with diameters in 2 progression - particle size distribution (number basis) over range 2 – 200 microns Frequency distribution curves corresponding to (a) a normal distribution, (b) a positively skewed distribution and (c) a bimodal distribution. | Slide 24 of 39 April 2007 API Properties – Particle Size Analysis Sieve analysis – equivalent diameters Sieve diameter ds for various shaped particles - ‘stack’ of sieves - particle size distribution (weight basis) over range 50 – 1000 microns | Slide 25 of 39 April 2007 API Properties Particle size, drug dissolution and bioavailability Dissolution is measure of rate of solution Dissolution related to particle size and particle surface area (smaller particle size, larger surface area, faster dissolution) dm kA C s C dt dm dt = dissolution rate, A = surface area of solid, k = dissolution rate constant, Cs = saturation of drug, C = concentration of drug in solution) | Slide 26 of 39 April 2007 API Properties – Particle Size Reduction Examples of drugs where a reduction in particle size has led to improvements in bioavailability | Slide 27 of 39 April 2007 API Properties – Biopharmaceutical Classification Scheme Valuable classification system to guide formulators in requirements for ‘particle engineering’ of APIs Consider aqueous solubility and permeability via oral route of delivery Class I – high solubility, high permeability - rapid absorption, good bioavailability - eg propanolol, metaprolol Class II – low solubility, high permeability - API controls absorption; potential for particle size effects on bioavailability - eg ketoprofen, carbamazepine | Slide 28 of 39 April 2007 API Properties – Biopharmaceutical Classification Scheme Class III high solubility, low permeability - APIs dissolve rapidly and poorly absorbed - require fast API dissolution to maximise absorption - potential benefits from particle size reduction eg ranitidine, atenolol Class IV low solubility, low permeability - challenging molecules, likely to exhibit low bioavailability eg hydrochlorothiazide, furosemide, - option to increase permeability - modify APIs as ‘prodrugs’ | Slide 29 of 39 April 2007 API Properties – Prodrugs with modified permeability and absorption Examples of prodrugs with improved permeability and oral absorption | Slide 30 of 39 April 2007 API Properties and Design of Medicines Wide range of dosage forms - liquids, semi-solids, solids Range of administration routes Medicines containing more than one API Single unit dosage and multi unit dose systems Device, administration and compliance issues All these are issues that can impose requirements for ‘desired’ API properties, in addition to chemical quality and safety assurance | Slide 31 of 39 April 2007 API Properties – Characteristics to be considered when formulating medicines | Slide 32 of 39 April 2007 API Property Classification – inter-dependencies between ‘groupings’ | Slide 33 of 39 April 2007 API Properties – Formulation Design and Processing Formulation design – dosage form and delivery route issues, and ‘functionality’ tests for guiding choice of processing route and conditions API stability, solubility (dissolution) and particle size are key properties for effective formulation design For preparation of solutions, suspensions, granules for reconstitution - NB attention to stability (chemical and physical) and storage requirements For solid dosage forms – eg tablets and capsules - NB biopharmaceutics classification - potential for increasing drug dissolution rate - potential for modifying drug solubility/permability (eg salts, prodrugs) | Slide 34 of 39 April 2007 API Properties – Formulation Design and Processing Additional tests being considered for including in pharmacopoeias as tests for APIs (and solid particle excipients) - these include ‘functionality’ based (to standardise ‘performance’ of API in formulation and secondary processing) - characterisation of crystalline and partially crystalline solids (by X-ray powder diffraction) - porosity and pore size distribution of solids (by mercury porosimetry) - water-solid interactions (by sorption isotherms, hygroscopicity, water activity) - particle size analysis (by laser light diffraction) - calorimetric and thermal behaviour of powders | Slide 35 of 39 April 2007 ‘Life-Time’ of APIs Appropriate specifications must be met throughout ‘life-time’ of API to ensure quality and safety Life-time = from - isolation of API - API received by product manufacturer from supplier - API processed into pharmaceutical product - storage period of product (shelf life limit) to - end of period of administration of product to patient NB Alternative specifications will apply at the different stages | Slide 36 of 39 April 2007 Challenges for API Procurement and Evaluation Compliance with CoA, and/or pharmacopoeial monograph Consistency within/between batches, sampling issues Alternative suppliers and CoG issues Building confidence in supplying agencies Quality and safety, quality and safety, quality and safety!! | Slide 37 of 39 April 2007 Challenges for API Formulation and Processing Identify critical chemical, physical and ‘functional’ properties which are crucial for specific formulation requirements Awareness of stability of API as pure substance, during formulation and processing, and through ‘shelf life’ of product This information needs to be linked to the type of dosage form required, route of administration and desired ‘shelf life’ of product under ‘anticipated’ storage conditions | Slide 38 of 39 April 2007 Physicochemical Properties of APIs their relevance to formulation Summary and conclusion Assurance of quality and safety of APIs Spectrum of tests and specifications of criteria for APIs Interdependency between ‘categories’ of API properties ‘Functionality’ testing related to formulation design (and processing route and conditions) Summary of ‘challenges’ in API procurement and in evaluating APIs for formulation design | Slide 39 of 39 April 2007