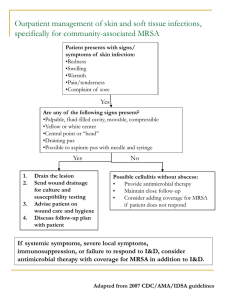
Antibiotic Choices - CriticalCareMedicine
advertisement

Antimicrobials Making sense of them W. Sligl Infectious Diseases/Critical Care Outline General considerations when prescribing antimicrobials Review of antibacterial classes Mechanisms of resistance Empiric Therapy Initiation of treatment for a clinical syndrome before the specific microbiology known ‘Best guess’ Requires some understanding of infectious disease epidemiology Case Examples Case #1: 17 yo M, previously healthy, with 2 day hx of fever, sore throat, cough. Diagnostic possibilities? Can he wait or should be be treated? What would you treat him with? Case #2: 17 yo M with advanced HIV and 2 day hx of fever, sore throat, cough. Diagnostic possibilities? Can he wait or should he be treated? What should he be treated with? Factors to Consider when Prescribing Antimicrobials Host Factors Microbial Factors Antimicrobial Factors Host Factors Age Immune adequacy Underlying diseases Renal/hepatic impairment Presence of prosthetic materials Ethnicity Pregnancy/nursing Age Can help to narrow the diagnosis with certain infections Examples: Meningitis What bugs would you consider in a neonate? In an adult? EBV infection In what age group would you consider this diagnosis? UTI How does age affect your interpretation of laboratory results? Immune Adequacy Immune status important May be at increased risk of specific infections Asplenia Encapsulated bacterial infections HIV/AIDS Opportunistic infections Transplantation Variety of infections depending on net degree of immunosuppression Underlying Diseases Increased risk of infection in pts with Diabetes mellitus HIV/AIDS Malignancies Renal impairment Autoimmune diseases Renal/Hepatic Impairment Implications for treatment Dose adjustments may be needed Avoid concomitant nephro- or hepato-toxic drugs May require fluid boluses prior to administration E.g. amphotericin B, acyclovir Implications for monitoring Monitor renal/liver function Consider monitoring drug levels if available (i.e. therapeutic drug monitoring; TDM) Presence of Prostheses Implications for diagnosis What bug(s) are more pathogenic in artificial joints/valves? Implications for treatment Infected hardware needs to be removed – antibiotics alone don’t usually work May add rifampin in certain situations (biofilm penetration) Ethnicity Consider diseases endemic in country of origin Examples: TB in patients from TB endemic areas as well as First Nations patients Strongyloides in patients from tropical countries Also consider malaria, trypanosomiasis, leptospirosis, leishmaniasis, leprosy Geographic Factors Need to know common microbial causes of infection in your area Example: Recent emergence of CA-MRSA in outpatient skin and skin structure infections Travel history is important Example: Fever in traveler returning from Sudan vs. person who has never left Edmonton Pregnancy and Nursing Safety of antibiotic use in pregnancy and nursing has to be considered Generally SAFE Beta-lactams Macrolides Clindamycin Conventional dosing AG NOT SAFE Fluoroquinolones TMP/SMX Extended interval AG Tetracyclines Microbial Factors Probable microorganisms Microbial susceptibility patterns Natural history of infections Likelihood of obtaining microbiologic data Site of Infection Probable Microorganisms Have to know most likely organisms for various common infections CAP, HAP, VAP Intra-abdominal infections Catheter-related UTIs Line infections Endocarditis Meningitis Microbial Susceptibilities Know general microbial susceptibilities as well as those which are geographically specific: S. pneumoniae ~15% resistant to erythromycin, ~3% to penicillin P. aeruginosa ~30-40% resistant to ciprofloxacin Higher in ICU pts; ~50-70% MRSA MRSA made up 6% of S. aureus isolates in Capital Health Region 2005 Huge increase in 2007-2008; up to 50% of isolates in OPAT setting (CA-MRSA) Vs. up to 70% in some US centers Know your local epidemiology! And don’t forget to ask about recent Abx use! Natural History of Infection Rapidly fatal vs. slow growing Meningococcemia – can be rapidly fatal TB meningitis often more indolent course Know what to expect Pyelonephritis – expect fever for up to 72 hours, image if still febrile after 72 hours to r/o perinephric abscess Likelihood of Obtaining Microbiologic Data May be difficult to get specimen(s) E.g. brain abscess If patient has been on antibiotics, will affect culture results May need to treat empirically, and follow clinical response/imaging Site of Infection Susceptibility testing is geared to attainable serum levels Does not account for host factors or conditions that alter antimicrobial access Diffusion into CSF is limited in many drugs Abscesses Difficult to penetrate abscess wall High bacterial burden Low pH and low oxygen tension can affect antimicrobial activity Antimicrobial Factors Route of Administration Bactericidal vs. bacteristatic Combination vs. monotherapy Route of Administration Many options exist Enteral Parenteral Nebulization Intrathecal Topical Enteral Administration Check drug oral bioavailability Must be resistant to breakdown by gastric acid Some drugs must be given with buffer Some require acidity for absorption Other drugs cannot be given in high enough doses orally (usually d/t side effects/intolerance) Bactericidal vs. Bacteristatic Antibiotics work by either killing (cidal) vs. halting growth (static) of microorganisms Cidal: beta-lactams, aminoglycosides, fluoroquinolones, glycopeptides, daptomycin, metronidazole Static: tetracyclines, macrolides, clindamycin, linezolid Combination Therapy Three main reasons: Broader coverage May be necessary for empiric treatment of certain infections E.g. intra-abdominal sepsis, VAP Synergistic activity E.g. amp + gent for serious enterococcal infections Prevent resistance E.g. TB, pseudomonal infection Disadvantages: Antagonism (e.g. linezolid and vancomycin) Potential for increased toxicity Adjunctive Approaches Don’t forget to do all the other stuff: Septic shock: EGDT, steroids, rhAPC Bacterial meningitis: steroids Benefit in S. pneumoniae in adults and H. influenzae in children Drainage and debridement of abscesses Removal of prosthetic materials Correction of malnutrition Assisted organ function Mechanical ventilation, CRRT/IHD, hemodynamic support with inotropes/vasopressors Monitoring Response to Therapy Monitor infectious parameters Fever WBC ESR etc. Know natural history Serial imaging may be useful Repeat cultures E.g. bacteremia, endocarditis Duration of Therapy Very few studies to establish minimum durations of therapy Duration usually based on anecdote Most uncomplicated bacterial infections should be treated for 7–14 days 10-14 days: bacteremia 4-6 weeks: endocarditis, empyema, septic arthritis, osteomyelitis 6-12 months: mycobacterial diseases, nocardia, endemic mycoses *VAP: used to recommend 14-21 days!; recent studies suggest 7-8 days is adequate but depends on microorganism, severity of disease, and pt comorbidities (e.g. may want to Rx immunosuppressed pts or those with Pseudomonas orS. aureus a little longer) Pharmacoeconomics Cost of illness includes Medications Provider visits Administration of medications Loss of productivity *Cost is a tertiary consideration after effectiveness and safety Antibacterials Beta-Lactams Includes Penicillins, cephalosporins, carbapenems, monobactams Mechanism of Action Inhibit cell wall synthesis by binding to PBP and preventing formation of peptidoglycan cross linkage Toxicity Hypersensitivity reactions Cross-reactivity with penicillin allergy 10-20% with carbapenems (50% if skin test +) 10% with 1st gen. cephalosporins 1% with 3rd gen. cephalosporins Beta-Lactams Natural Penicillins Includes Pen G, Pen V, benzathine penicillin Spectrum of activity Streptococci Viridans group strep, beta-hemolytic strep, many S. pneumoniae Most N. meningiditis Oral anaerobes Peptostreptococcus Other: Listeria monocytogenes, Pasteurella multocida, Treponema pallidum, Actinomyces israelii Aminopenicillins Prototypes: Ampicillin, Amoxicillin Spectrum of activity Streptococcus spp. Enterococcus faecalis (not faecium) Spectrum extended to include some GNB E. coli, Proteus mirabilis, Salmonella spp., Shigella, Moraxella, Hemophilus spp. Penicillinase Resistant Penicillins Prototype: Cloxacillin Spectrum of activity Staphylococci MSSA, 1/3 of CoNS Streptococcus spp. No enterococcal coverage No gram-negative or anaerobic coverage Carboxypenicillins Prototype: Ticarcillin Broad spectrum activity including Stenotrophomonas and Pseudomonas Problems with hypernatremia, hypokalemia, platelet dysfunction If clavulanate added – MSSA coverage, improved gram-negative and anaerobic coverage Ureidopenicillins Prototype: Piperacillin Spectrum of activity Streptococcus spp. (less than earlier generations) Enterococcus faecalis (NOT faecium) Anaerobic organisms Pseudomonas Broad Enterobacteraciae coverage If tazobactam added – MSSA coverage, improved gram-negative and anaerobic coverage Cephalosporins Divided into 4 generations In general: ↑ gram-negative coverage and ↓ gram-positive coverage with ↑ generation Enterococci not covered by any generation! 1st Generation Prototype: Cefazolin (Ancef®) Spectrum of activity MSSA Streptococcus spp. E. coli, Klebsiella, Proteus mirabilis No anaerobic activity 2nd Generation Prototype: Cefuroxime Spectrum of activity Gram positives (MSSA, Streptococcus) H. influenzae M. catarrhalis 3rd Generation Divided into two main groups: Ceftazidime Ceftriaxone and cefotaxime Pseudomonal coverage Good gram-negative coverage Less gram-positive coverage Less reliable MSSA coverage Good gram-negative coverage No anti-pseudomonal activity No anaerobic activity Good CSF penetration – used in meningitis Toxicity includes biliary sludging Cefixime – oral equivalent No anti-pseudomonal activity 4th Generation Cefepime Maintains gram positive activity, better MSSA coverage than with 3rd generation cephalosporins Active against Pseudomonas ? Activity against SPICEM organisms Lower potential for resistance The Next Generation “Fifth Generation”; “Extended-spectrum” Ceftobiprole (Zeftera) Recently approved by Health Canada (June 2008) Available via special access First broad-spectrum anti-MRSA cephalosporin Broad-spectrum activity including MRSA, Pseudomonas, and E. faecalis Reduced activity against cephalosporin-resistant SPICEM and ESBLs organisms Binds to PBP2a (MRSA) and PBP2x (penicillinresistant S. pneumoniae) The Next Generation “Fifth Generation”; “Extended-spectrum” Ceftobiprole (Zeftera) Caramel taste during infusion (diacetyl formed during conversion from prodrug to active metabolite) Statistically non-inferior to vancomycin and vancomycin/ceftazidime for the treatment of skin and soft tissue infections STRAUSS 1 and 2 trials Current indications: complicated skin and skin structure infections (cSSSI), DM foot infections, ?nosocomial pneumonia (awaiting further trials) Dose: 500mg IV q12h for GP, 500mg IV q8h for GN Carbapenems Imipenem/Meropenem MSSA, Streptococcus Broad-spectrum gram-negative coverage including SPICEM organisms Pseudomonas Enterococcus faecalis but NOT faecium Anaerobic activity Ertapenem Allows once a day dosing Does not cover Pseudomonas or Enterococcus Monobactam Prototype: Aztreonam Aerobic GNB Including Pseudomonas No gram-positive or anaerobic coverage Similar spectrum to aminoglycosides without renal toxicity Cross reactivity to penicillin rare (may use in pen-allergic pts) Some cross-reactivity with ceftazidime (same side-chains) Aminoglycosides Includes Gentamicin Tobramycin Amikacin Streptomycin Mechanism of action Bind to 30S/50S ribosomal subunit Inhibit protein synthesis Toxicity CN VIII - irreversible Renal toxicity – reversible Rarely hypersensitivity reactions Aminoglycosides Spectrum of activity Aerobic GNB including Pseudomonas Mycobacteria (mainly streptomycin) Brucella, Fransicella (tularemia) Nocardia Synergistic with beta-lactams (Enterococci, Staphylococci) Fluoroquinolones Includes Ciprofloxacin Ofloxacin Levofloxacin Gatifloxicin Moxifloxacin Mechanism of Action DNA gyrase inhibitors Toxicity GI symptoms, QTc prolongation Fluoroquinolones All cover Atypicals: Mycoplasma, Legionella, Chlamydia Fransicella, Rickettsia, Bartonella Atypical mycobacteria Ciprofloxacin Good gram-negative coverage N. gonorrhea, H. influenzae Good for UTI, infectious diarrhea May be used in combination for Pseudomonas Fluoroquinolones Levofloxacin L-enantomer of ofloxacin Better gram-positive coverage (mainly Streptococcus) than ciprofloxacin Used for LRTI Moxifloxacin Quite broad-spectrum Activity against Strep/Staph plus gram-negatives Anaerobic coverage Minimal to no anti-pseudomonal activity Macrolides Includes Erythromycin Clarithromycin Azithromycin Mechanism of Action Bind to ribosomal subunit Block protein synthesis ***Static, not cidal Toxicity GI upset (especially with erythromycin) Erythromycin Active against Streptococcal spp. Also effective against Legionella Mycoplasma Campylobacter Chlamydia Neisseria gonorrheae Poor for H. influenzae Used infrequently due to GI upset Lots of safety data in children/pregnancy Clarithromycin Spectrum of activity Streptococci including S. pneumoniae Moraxella, Legionella, Chlamydia Atypical mycobacteria More active against H. influenzae Used in combination against H. pylori Less GI side effects Azithromycin Spectrum of activity Mycoplasma, Legionella, Chlamydia H. influenzae Streptococcus spp. Long half-life 5 day course is adequate Less GI side effects Anti-inflammatory properties in addition to antimicrobial action? Some evidence of improved outcomes when added to beta-lactam in bacteremic pneumococcal CAP Telithromycin Ketolide, similar to macrolides Macrolide resistant S. pneumoniae usually the result of a point mutation altering ribosomal target site binding Telithromycin binds to two independent sites on 50S and is a poor substrate for efflux – potent against macrolide-R pneumococcus Bewrare serious side effects: hepatic necrosis, GI upset, arrhythmias, rash *P450 inhibitor – multiple drug interactions Used only for mild-moderate CAP due to multidrug resistant S. pneumoniae Clindamycin Mechanism of Action Blocks protein synthesis by binding to ribosomal subunits ***Static, not cidal Toxicity Rash GI symptoms C. difficile colitis in 1-10% Covers MSSA, Streptococcus, and anaerobes No gram-negative or Enterococcal coverage Tetracyclines Includes Tetracycline Doxycycline Minocycline Tigecycline (glycylcycline) Mechanism of Action Bind to 30S ribosomal subunit Block protein synthesis ***Static, not cidal Toxicity Rash, photosensitivity, impairs bone growth and stains teeth of children, increased uremia Tetracyclines Spectrum includes unusual organisms Rickettsia Chlamydia Mycoplasma Vibrio cholera Brucella Borreila burgdorferii (Lyme disease) Minocycline Active against Stenotrophomonas and P. acnes May be active against MRSA Doxycycline Used in uncomplicated CAP and for prophylaxis against malaria Tigecycline Tygacil® Novel broad-spectrum Glycylcyline Biliary/fecal excretion Active against gram-positives including MSSA and MRSA (not VRE), Enterobacteraciae including ESBLs, MDR-Acinetobacter, and anerobes No anti-pseudomonal activity For complicated intra-abdominal and skin/soft tissue infections Not approved for bacteremia or pneumonia Glycopeptides Prototype: Vancomycin Mechanism of Action Inhibits cell wall synthesis Toxicity Ototoxicity – rare Can induce histamine release – red man syndrome Usually with rapid infusion Glycopeptides Spectrum of activity Gram-positives: S. aureus (incl. MRSA), CoNS, Streptococcus, Enterococcus Gram-positive anaerobes Exceptions: VRE, Leuconostoc, Lactobacillis Inferior to beta-lactams in terms of cure rates for beta-lactam sensitive organisms Big, bulky molecule – poor CSF penetration in the absence of meningeal inflammation (including those treated with corticosteroids) Lipopeptides Daptomycin or Cubicin® Bactericidal Disrupts bacterial membrane function Binds to cell membrane, forms ion channel, K+ efflux, depolarization, cell death In vitro activity against gram-positive organisms including MSSA, MRSA, VRSA, VRE, PRSP Daptomycin Approved for use in MSSA/MRSA and other selected gram-positives (not VRE, yet): Complicated skin and soft tissue infections S. aureus bacteremia/R. IE Cannot be used to treat pneumonia Does not achieve sufficiently high concentrations in the respiratory tract Inactivated by surfactant Side effects: myopathy; monitor CK Metronidazole Mechanism not well understood Interferes with DNA synthesis via toxic intermediates Spectrum of activity Most anaerobes except Peptostreptococcus, Actinomyces, Propionibacterium acnes Parasites: Giardia lamblia, Entamoeba histolytica Toxicity Disulfuram reaction Neuropathy Potentiation of warfarin Sulfa drugs Includes: TMP/SMX Mechanism of Action Folate reductase inhibitor Toxicity Hypersensitivity reactions Thrombocytopenia Rash Hyperkalemia Sulfa drugs Broad-spectrum coverage Streptococcus, Staphylococcus H. influenza L. monocytogenes Many Enterobacteraciae (E. coli, Klebsiella) Stenotrophomonas maltophila PJP Nocardia Isospora belli Frequent allergic rxns Used in special circumstances (e.g. PJP, nocardia, Stenotrophomonas) Chloramphenicol Broad-spectrum activity GPC, GNB Meningitis organisms Rickettsia spp. No activity against Klebsiella, Enterobacter, Serratia, Proteus, Pseudomonas Toxicity Dose related marrow toxicity Idiosyncratic aplastic anemia Gray baby syndrome Linezolid Oxazolidinone Binds to ribosomal subunit inhibiting protein synthesis Static, not cidal Excellent oral bioavailability Active against MSSA, MRSA, enterococci including VRE, S. pneumoniae No activity against gram-negatives Linezolid No cross-resistance with other drugs Approved for use in nosocomial pneumonia and skin/soft tissue infections Major side effect: Reversible myelosuppression Monitor CBCD Resistance reported, but rare Very expensive ($140/day) and currently not covered (used mainly in WCB cases) Quinupristin/dalfopristin Synercid® Combined stretogramin A and B Bactericidal Approved for use in skin and soft tissue infections only Active against a wide variety of gram-positive bacteria MSSA, MRSA, CoNS, Streptococci, VRE (E. faecium not E. faecalis) Major side effect: phlebitis, hyperbilirubinemia Resistance, although rare, has been reported Colistin Polymyxin E Older drug, recently has come into re-use Binds to phospholipids in cell membrane causing disruption Most commonly used in salvage Rx in MDRPseudomonas or Acinetobacter infections Bactericidal Nephrotoxic, neurotoxic *Needs to be renally adjusted Nitrofurantoin Synthetic nitrofuran Inhibits bacterial acetylcoenzyme A – disrupts carbohydrate metabolism Cidal at high concentrations, static at lower Concentrated in urine with normal renal function Contraindicated in renal insufficiency Nitrofurantoin Effective against: E. coli, Enterococcus, S. aureus, some strains Klebsiella and Enterobacter Proteus, Serratia, and Pseudomonas are resistant! *Only indication is Rx or prophylaxis of lower UTIs Should not be used for systemic infection Adverse effects: n/v, hypersensitivity pneumonitis, pulmonary fibrosis, hemolytic anemia in G6PD deficiency, hepatitis Mechanisms of Action Summary I Mechanisms of Action Summary II Mechanisms of Resistance Mechanisms of Resistance Enzymatic inactivation of antimicrobial Target site binding Efflux Decreased permeability Others Enzymatic Inactivation Beta-lactamases Penicillinases, ampCs, ESBLs, metallo-betalactamases Seen in S. aureus, H. influenzae, N. meningitidis, SPICEM, E. coli, Klebsiella spp., P. aeruginosa Enzymatic Inactivation AG-modifying enzymes n-acetylation, o-nucleotidylation, o-phosphorylation Seen in Enterobacteriaceae, Pseudomonas, and Enterococci Macrolide, lincosamide, and streptogramin inactivating enzymes (esterases) Uncommon Altered Target Site Binding Cell wall precursor targets D-ala-D-ala changed to D-ala-D-lac in VRE Target enzymes PBP2a in MRSA; low affinity for betalactams Ribosomal target sites Methylase enzymes Seen in tetracyclines, macrolides, lincosamides, aminoglycosides erm gene confers MLSB phenotype in S. aureus which may be constitutive or inducible Efflux Seen with tetracyclines, macrolides, streptogramins, beta-lactams, fluoroquinolones, and carbapenems Macrolide resistance in S. pneumoniae (mef) Staphylococci (msr) Beta-lactam resistance in Pseudomonas Fluoroquinolone resistance in Enterobacteriaceae Clindamycin Resistance ERYTHRO CLINDA Decreased Permeability Porin channels determine rate of diffusion of Abx – mainly a problem in GN organisms Causes Fluoroquinolones resistance in: P. aeruginosa and S. marsecans Aminoglycoside resistance in: E. coli, S. aureus, and Salmonella spp. Others Target site protection DNA gyrase protection and fluoroquinolone-R Ribosomal protection and tetracycline-R Overproduction of target Sulfonamides compete with enzyme DHFR and halt nucleic acid production Overproduction of DHFR may overwhelm sulfa inhibition Bypass of antimicrobial inhibition Development of different growth factor requirements and subsequent evasion of inhibition E.g. trimethoprim/sulfamethoxazole Questions?