Heat and States of Matter
advertisement
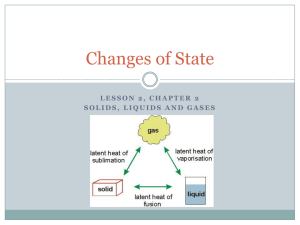
Chapter 9: Heat and States of Matter Section 2: States of Matter States of Matter 2 Four States of Matter SOLID STATE • The particles of a solid are packed closely together and are constantly vibrating in place. • The attractions between particles are strong and solids have a fixed volume and shape. States of Matter 2 LIQUID STATE • The attractive forces are strong enough to cause particles to cling together. • Liquids have a definite volume, but not a definite shape. States of Matter 2 GAS STATE • In a gas the forces between particles are so weak that the particles no longer cling together. • Gases do not have a definite shape or volume. States of Matter 2 Plasma State • The most common state of matter in the universe is the plasma state. • PLASMA is matter consisting of positively and negatively charged particles and does not have a definite shape or volume. States of Matter 2 Changing States Melting • The temperature at which a solid begins to melt is its melting point. • The amount of energy required to change 1 kg of a substance from a solid to a liquid at its melting point is known as the HEAT OF FUSION. States of Matter 2 Freezing • The HEAT OF FUSION is also the energy released when a liquid freezes. • The attractive forces are strong enough that the particles form an ordered arrangement. States of Matter 2 Vaporization • Vaporization occurs as liquid changes into a gas. • Vaporization that occurs at the surface of a liquid is called evaporation. • Evaporation causes the temperature of the liquid to decrease. • Can you explain this? States of Matter 2 Boiling • The BOILING POINT of a liquid is the temperature at which the pressure of the vapor in the liquid is equal to the external pressure acting on its surface. • The HEAT OF VAPORIZATION is the amount of energy required for 1 kg of the liquid at its boiling point to become a gas. States of Matter 2 CONDENSATION • The HEAT OF VAPORIZATION is also the amount of energy released during condensation. • This graph shows the temperature change of water as thermal energy is added. States of Matter 2 CONDENSATION • The average kinetic energy of the water molecules doesn’t change. • The temperature remains constant during melting. What about during evaporation? States of Matter 2 CONDENSATION • After the liquid water has changed completely into a gas, the temperature of the gas increases as energy is added. States of Matter 2 Thermal Expansion THE THERMAL EXPANSION OF MATTER • The increased separation between the particles results in the expansion of the object and the size of the object increases. • When a material cools, the particles in the material move more slowly and become closer together. 2 States of Matter THERMAL EXPANSION OF LIQUIDS • The forces between the particles in liquids are weaker than the forces between the particles in a solid. • The same temperature increase usually causes liquids to expand much more than solids. 2 States of Matter THERMAL EXPANSION OF GASES • In a gas, the forces between particles are much weaker than they are in liquids. • Gases expand even more than liquids for the same increase in temperature. Section Check 2 Question 1 _________ is a state of matter consisting of positively and negatively charged particles that exists where the temperature is extremely high. A. B. C. D. Gas Liquid Plasma Solid Section Check 2 Answer The answer is C. Plasma is found in the Sun, stars, lightning bolts and neon lights. Section Check 2 Question 2 Most __________ materials have a specific type of geometric arrangement. A. B. C. D. gaseous inert liquid solid Section Check 2 Answer The answer is D. The particles in most solids align themselves in ordered geometric patterns. Section Check 2 Question 3 The amount of energy required to change 1 kg of a substance from a solid to a liquid at its melting point is known as the _______. A. B. C. D. heat of energy heat of fusion heat of melting heat of vaporization Section Check 2 Answer The correct answer is B. Heat of fusion causes an ice cube to become liquid water.