Thermodynamics_Syllabus - The University of Tennessee at
advertisement

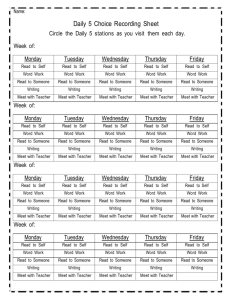
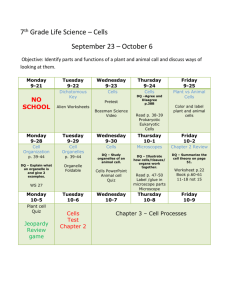
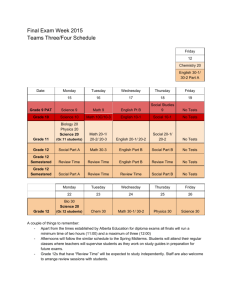
GEOG 440- Atmospheric Thermodynamics University of Tennessee at Martin Department of Agriculture, Geosciences & Natural Resources Course Syllabus, Fall 2013 Instructor: Office: Email: Office Hours: Chris Karmosky 201-C Johnson EPS ckarmosk@utm.edu 11:00-12:00 MTWTh, otherwise by appointment (email: ckarmosk@utm.edu) Course Time: MTWTh 3:00 to 3:50 Room: 227 EPS Course Prerequisites: GEOG 305 (Principles of Meteorology), MATH 252 (Calculus II, may be taken concurrently with instructor’s permission) Required Text: Atmospheric Thermodynamics (Bohren and Albrecht) Why would I want to take this course? This course is the first of two courses that examine the physics of the atmosphere. Atmospheric Thermodynamics focuses on the laws of thermodynamics, the role of moisture and changes of state, thermodynamic diagrams, and atmospheric stability. Evaluation: The grade for this course will be based on weekly problem sets, four midterm exams, and a final exam. The point breakdown is as follows: Four Midterm Exams (100 Points Each), Lowest dropped Thirteen Problem Sets (25 Points each), Lowest three dropped Final Exam (100 Points) Total: Approximately 650 Points Students are expected to take all quizzes, midterms, and the final exam on the scheduled dates. For this course, I will drop the lowest exam grade and lowest two problem set grades. No make-ups will be administered; exams where students must be absent will count as the dropped exam. Unless otherwise stated, all projects are due at 5PM on the due date at the top of the assignment. Electronic submissions of typed work will be accepted. Late work will be accepted, but only for partial credit. A deduction of 10% per day (up to a maximum deduction of 50%) of the point value will apply for late work. No late work will be accepted after December 6. It is your personal responsibility to: adhere to the guidelines set forth in this syllabus, keep up with impromptu changes, study, and complete all assignments and exams. Attendance: Class time is my primary communication of revised deadlines, changes to the syllabus, and is the primary time that I distribute course handouts and assignments. While such handouts and assignments are usually also disseminated via email and/or blackboard, it is not the instructor’s responsibility to provide electronic copies of materials to you if you were not present in class when they were distributed. Students are responsible for all course material and are expected to participate in classroom activities and discussion. I often scoff at the question “Did I miss anything important?” because if I didn’t feel it were important, I wouldn’t cover it in class. Furthermore, because attendance is an expectation, not “going above and beyond”, I do not give extra credit points or have an attendance portion factored into my grading scheme. I do, however, as discussed below, deduct points if students fail to attend class consistently. If you cannot attend class for some reason, you are personally responsible for the information covered. ***I will be happy to discuss what was covered, but it is NOT my responsibility to provide you with a detailed overview or a set of notes.*** After two unexcused absences, one percentage point will be deducted from your total course grade for each additional two unexcused absences. Religious Holidays: I will make every reasonable effort to allow students to observe their religious holidays without penalty. If you must miss class, however, it is still your responsibility to make up any missed material. Please let me know the dates you will miss well ahead of time. Academic Honesty: Academic honesty and integrity is expected in this course (and any other course at UTMartin for that matter). You are expected to produce work that is entirely your own for each and every assignment. All quizzes and exams are closed-note, closed-book, and closed-neighbor. Take-home assignments must also be your own work. While asking questions regarding the subject material is permissible, copying and/or paraphrasing another person’s work will be considered plagiarism. Plagiarism includes, but is not limited to: Copying sections (or even single lines) of another person’s work without attribution. Using a line from another person’s work, and citing it, but not putting it into quotes or your own words Inserting a quote without appropriate context—at that point you are turning in the other person’s work, not your own Using information that is not “basic knowledge” from a source without citing the source, even if you put that information into your own words Writing facts and figures without attributing their source Facilitating academic dishonesty is also considered academic dishonesty. Again, operating by the “better safe than sorry” principle works best, and I would encourage students who feel uncomfortable giving assistance to a student to refer that student to me for assistance. While I may not be able to detect each and every instance of plagiarism, know that this is something I take seriously, and I have assigned failing grades for assignments where plagiarism has occurred in the past. For me to do otherwise would be unfair to the majority of students who are honest. In the world outside of the university, representing another person’s work as your own is something that can get you fired from a job and can incur fines and prosecution depending on the severity. Any academic dishonesty will result in a failing grade for the assignment, and will be reported to the Division of Student Affairs. Classroom Etiquette: 1. Cell phones should be turned off or set to vibrate when entering the class. Text messaging is NOT allowed during class at any time. If you receive a call or message, ignore it until class is over (or leave the room if the call is an emergency) 2. Texting, consulting notes, or consulting electronic material of any sort during exams is considered a violation of academic integrity. This includes looking at a phone, iPod or tablet to check the time. You will be asked to turn in your paper immediately, receive a “0” for that exam, which may lead to failing the course. Operating by the “better safe than sorry” principle is expected. If you opt to take a call or message and leave the room during any graded (closed-book) activity, you will NOT be allowed to continue. You will be required to turn in the paper immediately. IF you find yourself entering an exam with the expectation of an urgent message that cannot wait for the end of the session (e.g., family medical situation that could change at any moment), please inform me of that issue prior to class. Depending upon the situation, I may be able to make suitable arrangements. 3. Refrain from excessive talking as it disrupts the normal learning environment. If you desire to engage in such behavior, you may be asked you leave the room. 4. Laptops and tablets are not generally allowed to be used in the classroom, though if special circumstances arise, please consult with me. Web surfing or instant messaging during class will not be tolerated—you will be dismissed from the class and recorded as an unexcused absence. 5. Please inform your instructor when you may have to arrive late or leave early, preferably in advance. 6. Be patient and courteous to other students and your professor. Courtesy includes having a good attitude, paying attention (listening to me when I am speaking and others when they ask a question), even when the subject may be obvious to you. 7. The classroom operates under a “Hats Off” policy during exams and sunglasses should not be worn in the classroom; absolutely not during examinations. 8. Communication between students and instructors via email is common. Emails should be written in standard English, complete with appropriate punctuation. Emails written in “text speak” will not be read. Student Success Center, Office of Disability Services: If you require additional accommodations as the result of a disability, it is your responsibility, within the first two weeks of class, to coordinate with the Office of Disability Services as per the guidelines below. Students have the following roles in the academic accommodation process: Identify themselves to the Office of Disabilities Services if they need accommodations; Provide documentation of their disability to the Office of Disabilities Services; Participate with the Office of Disabilities Services in the interactive process of determining and implementing reasonable accommodations; Make arrangements for accommodations by providing their professors with a letter from the Office of Disabilities Services approving accommodations and work directly with the professors and staff involved in the provision of an approved accommodation; and Inform the Office of Disabilities Services when accommodations are not provided, accommodations are not working, accommodations need to be modified, or symptoms change. I will work within the guidelines suggested by the student success center, and will take all reasonable actions necessary to provide necessary accommodations. Please visit the following page for more information: http://www.utm.edu/departments/success/disability.php Writing Center: The Hortense Parrish Writing center is a resource for any student who would like individualized help in improving their writing. The Writing Center is open to students for tutoring, computer use, printing, workshops, reading, and general study, and I encourage you to take advantage of this resource! Their web URL is below: http://www.utm.edu/departments/writingcenter/ ***I reserve the right to make changes to the syllabus as we go along. You will still be responsible for the changes announced in class*** Course Schedule: (Assigned Readings in Italics) Week 1: Monday (8/26) Introductions and Expectations , Review of systems and thermodynamic properties (pp. 21-22) Week 2: Week 3: Tuesday (8/27) Conservation of Energy (pp. 1-10) Wednesday (8/28) Kinetic Energy Exchanges, Working and Heating (pp. 10-21) Thursday (8/29) Thermodynamic Energy and the First Law (pp. 22-28) **Assign Problem Set #1 Monday (9/2) Labor Day Holiday (No Class) Tuesday (9/3) Ideal Gas Law (pp. 34-44) Wednesday (9/4) Ideal Gas Law, cont. (pp.44-54) Thursday (9/5) Pressure decrease with height (pp. 54-66) **Problem Set #1 Due, Assign Problem Set #2 Monday (9/9) Intermolecular separation, Mean Free Path, Collision Rate (pp. 6671) Tuesday (9/10) Pressure Gradient, Surface Pressure, Dalton’s Law (pp. 71-78) Wednesday (9/11) Specific Heats and Enthalpy (pp. 99-106) Thursday (9/12) Poisson’s Relations, Dry Adiabatic Lapse Rate (pp. 106-111) **Problem Set #2 Due, Assign Problem Set #3 Week 4: Week 5: Week 6: Monday (9/16) Stability and buoyancy (pp. 111114) Tuesday (9/17) Review for Exam #1 Wednesday (9/18) Midterm Exam #1 Thursday (9/19) Specific Heats of Gas Molecules (pp. 114-122) **Problem Set #3 Due, Assign Problem Set #4 Monday (9/23) Heat capacities of mixtures of gases (pp. 123-127) Tuesday (9/24) Entropy (pp. 135-145) Wednesday (9/25) The second law and stability (pp. 145-154) Thursday (9/26) Entropy changes of liquids and solids, Potential Temperature (pp. 155-161) **Problem Set #4 Due, Assign Problem Set #5 Monday (9/30) Atmospheric Applications of the Second Law (pp. 161-171) Tuesday (10/1) The Carnot cycle (pp. 171-177) Wednesday (10/2) Water and its transformations (pp. 181-185) Thursday (10/3) Measures of water vapor in air (pp. 185-192) **Problem Set #5 Due, Assign Problem Set #6 Week 7: Week 8: Week 9: Week 10: Monday (10/7) The Clausius-Clapeyron Equation (pp. 192-204) Tuesday (10/8) Review for Exam #2 Wednesday (10/9) Midterm Exam #2 Thursday (10/10) The Clausius-Clapeyron Equation cont’d. (pp. 192-204) **Problem Set #6 Due, Assign Problem Set #7 Monday (10/14) Fall Break (No Class) Tuesday (10/15) Fall Break (No Class) Wednesday (10/16) van der Waals equation of state (pp. 204-218) Thursday (10/17) van der Waals equation of state, cont’d. (pp. 204-218) **Problem Set #7 Due, Assign Problem Set #8 Monday (10/21) Phase Diagrams (pp. 218-223) Tuesday (10/22) Free Energy (pp. 223-226) Wednesday (10/23) Effect of air pressure on Saturation Vapor Pressure (pp. 226-233) Thursday (10/24) Air in Water: Henry’s Law (pp. 233-238) **Problem Set #8 Due, Assign Problem Set #9 Monday (10/28) Size dependence of vapor pressure (pp. 238-252) Week 11: Week 12: Tuesday (10/29) Vapor Pressure of Solution Droplets (pp. 252-256) Wednesday (10/30) Precipitable water in the Atmosphere, Lapse Rate of the Dewpoint (pp. 272-278) Thursday (10/31) Density of Moist Air: Virtual Temperature, Wet-Bulb Temperature (pp. 278-287) **Problem Set #9 Due, Assign Problem Set #10 Monday (11/4) Review for Exam #3 Tuesday (11/5) Midterm Exam #3 Wednesday (11/6) Isentropic Ascent of a Saturated Parcel (pp. 287-299) Thursday (11/7) Thermodynamic Diagrams (pp. 299-311) **Problem Set #10 Due, Assign Problem Set #11 Monday (11/11) Thermodynamic Diagrams, cont’d. (pp. 299-311) Tuesday (11/12) Stability and Cloud Formation (pp. 311-322) Wednesday (11/13) Mixing Clouds, Cloud Formation on Ascent and Descent (pp. 322327) Thursday (11/14) Energy Transfer (pp. 335-347) **Problem Set #11 Due, Assign Problem Set #12 Week 13: Week 14: Week 15: Week 16: Monday (11/18) Energy Transfer, cont’d. (pp. 347356) Tuesday (11/19) Energy Transfer: Radiative, Convective (pp. 356-366) Wednesday (11/20) Momentum Transfer (pp. 366-372) Thursday (11/21) Mass Transfer: Diffusion (pp. 372379) **Problem Set #12 Due, Assign Problem Set #13 Monday (11/25) Review for Midterm Exam #4 Tuesday (11/26) Midterm Exam #4 Wednesday (11/27) Thanksgiving Holiday (No Class) Thursday (11/28) Thanksgiving Holiday (No Class) Monday (12/2) Selected topics in Atmospheric Dynamics, Readings TBA Tuesday (12/3) Selected topics in Atmospheric Dynamics, Readings TBA Wednesday (12/4) Selected topics in Atmospheric Dynamics, Readings TBA Thursday (12/5) Selected topics in Atmospheric Dynamics, Readings TBA **Problem Set #13 Due Final Exam: Wed. Dec. 11, 12:45-2:45PM Please refer to http://www.utm.edu/departments/registrar/final.php for any changes regarding the final exam scheduling.