Cu + + + + + + + + + + + + + + + + + + + +
advertisement

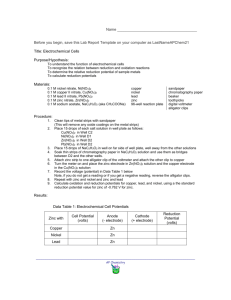
A simple chemical cell can be set up using copper and zinc electrodes. Here, we’ll show you how it works. containers We’ll construct this cell. We start with two empty containers… copper electrode We place a strip of copper metal in one of them. We call this a copper electrode. copper electrode Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu A metal is composed of neutral atoms. We’ll show (click) a few neutral copper atoms on this electrode. zinc electrode Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu In the other container, we’ll place a piece of zinc metal, which we call the zinc electrode. zinc electrode Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Cu Cu We’ll show a few neutral zinc atoms (click) on this electrode. Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn A ammeter Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn We obtain an ammeter. A ammeter measures the rate of flow of electrons, or current. A – – + + wires Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn We’ll attach the ammeter to each electrode using conducting wires. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Wires are –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu composed of neutral atoms Zn Cu Zn Cu Zn Cu Zn Zn Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn wires are composed of neutral atoms, which have the same number of protons as electrons. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ protons Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Because protons are positive, we’ll represent protons in the wires by positive charges A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ electrons Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn And electrons are negative, so we’ll represent electrons by negative charges. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ protons are in + + + – – – fixed positions Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Protons in all materials are in fixed positions in the nuclei of atoms, so they don’t move in the wires. –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu A – – – – – – – – – – – – – – – – – – – + + + + + + + + + + + + + + + + + + + electrons Cu Zn Cu Zn Cu Zn Cu Zn Cu Cu But in metals, electrons can move easily Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu Zn Cu Zn Zn Cu Zn Cu + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Cu(NO3)2 In the container with the copper electrode, we add some copper(II) nitrate solution A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 Zn NO3 Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu(NO3)2 Copper(II) nitrate consists of (click) copper 2 plus ions and (click) nitrate ions. These ions are free to move around in the solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 Cu Zn Cu 2 Cu Cu 2 Cu NO3 Zn NO3 Cu 2 NO3 Zn NO3 +6 Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 The three copper 2+ ions we’ve represented here, have total charge of positive 6. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 Cu Zn Cu 2 Cu Cu 2 Cu NO3 Zn NO3 Cu 2 NO3 Zn NO3 –6 Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 And the six nitrate ions we’ve represented have a total charge of negative 6, so at this point, charges are balanced in this solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 Zn NO3 Zn + + + Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu(NO3)2 – – – Zn(NO3)2 In the beaker with the zinc electrode, we add zinc nitrate solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 NO3 NO3 Zn Zn NO3 Cu(NO3)2 Zn 2 + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 Zinc nitrate solutions consists of (click) zinc 2 plus ions and (click) nitrate ions A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 NO3 NO3 Zn +2 NO3 Cu(NO3)2 Zn 2 Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 The zinc 2+ ion we’ve represented here has a charge of positive 2. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 NO3 NO3 Zn –2 NO3 Cu(NO3)2 Zn 2 Zn + + + – – – Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 And the two nitrate ions we’ve represented have total charge of negative 2. So at this point, charges are also balanced in this solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ + + + – – – KNO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Cu Zn Cu NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu Zn NO3 Cu 2 NO3 NO3 NO3 Zn Zn NO3 Cu(NO3)2 Zn 2 Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 Between the two containers we add (click) an inverted U tube with a solution of a salt like potassium nitrate, KNO3. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ + + + – – – KNO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Zn Zn NO3 NO3 Cu Zn Cu 2 Cu Cu 2 Cu NO3 Zn salt bridge NO3 NO3 Cu 2 NO3 Zn NO3 Cu(NO3)2 We call this a salt bridge. Zn Zn 2 Zn(NO3)2 NO3 Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ K+ KNO3 NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 Cu 2 Cu Cu 2 NO3 salt bridge K+ Zn NO3 K+ Zn K+ NO3 Zn NO3 NO3 NO3 Cu 2 Cu(NO3)2 Zn Zn NO3 NO3 – – – K+ Zn NO3 Cu Cu NO3 K+ Cu + + + Zn 2 Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 KNO3 solution consists of K plus and NO3 minus ions. Like all ions in solutions, these ions are free to move. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 Cu 2 Cu Cu 2 NO3 salt bridge K+ Zn NO3 Zn Zn NO3 Cu Cu NO3 K+ Cu K+ K+ NO3 Zn NO3 NO3 NO3 Cu 2 Cu(NO3)2 Zn Zn NO3 NO3 – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 Now we’ll focus on the zinc electrode and have a look at one of the zinc atoms. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 Cu 2 Cu Cu 2 NO3 salt bridge K+ Zn NO3 Zn Zn NO3 Cu Cu NO3 K+ Cu K+ –– Zn Zn K+ NO3 2 NO3 NO3 NO3 Cu 2 Cu(NO3)2 Zn Zn NO3 NO3 – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn NO3 Zn(NO3)2 This zinc atom loses two electrons and changes into a zinc 2 plus ion, as shown by the equation Zn gives Zn2+ plus 2 electrons. This ion then (click) leaves the metal and is dissolved in the solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 Cu Cu 2 salt bridge K+ Zn NO3 Cu 2 K+ Zn K+ NO3 Zn 2 NO3 NO3 Cu(NO3)2 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu 2 – – – –– NO3 Zn NO3 Cu Cu NO3 K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 The electrons supplied by the zinc push the other electrons through the wire (click twice while watching) A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Zn Zn2+ + 2e– –+ K K – + + NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 Cu Cu 2 salt bridge K+ Zn NO3 Cu 2 K+ Zn K+ NO3 Zn 2 NO3 NO3 Cu(NO3)2 Zn Zn NO3 Zn 2 Zn(NO3)2 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu 2 – – – – NO3 Zn NO3 Cu Cu NO3 K+ Cu + + + NO3 Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Zn Zn2+ + 2e– –+ K K – NO NO – K 3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu salt bridge Zn NO3 Zn Zn NO3 Cu 2 Cu 2 Cu NO3 NO3 Cu Cu 3 + K+ Cu K+ K+ NO3 Zn 2 NO3 NO3 Cu(NO3)2 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu 2 – – – + + Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 Meanwhile, over at the copper electrode, a copper 2+ ion moves to the surface of the electrode. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Zn Zn2+ + 2e– –+ K K – NO NO – K 3 Cu Cu Cu Cu Cu Cu Cu Cu salt bridge Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 NO3 Cu 2 NO3 Cu 2 NO3 Cu(NO3)2 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 2 Cu Cu Cu NO3 NO3 Cu Cu 3 + K+ Cu – – – + + Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 The two extra electrons on the copper electrode (click), move onto the Copper 2+ ion A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu K+ Cu NO3 Cu Cu NO3 Cu –Cu Cu Cu NO3 salt bridge K+ Zn K+ K+ NO3 NO3 NO3 Cu 2 NO3 Cu(NO3)2 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu 2 Cu Zn 2 NO3 2 Cu Zn NO3 Zn NO3 – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 The copper 2+ ion gains these electrons and changes into a neutral copper atom, as shown by the equation Cu2+ plus 2 electrons forms Cu. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 NO3 Cu 2 NO3 Cu 2 NO3 Cu(NO3)2 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 This whole process keeps repeating itself, causing electrons to continuously move through the ammeter. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 Cu(NO3)2 Now, we’ll focus on the ions in the container with the copper(II) nitrate solution A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 NO3 Cu 2 Cu 2 NO3 NO3 Zn Zn NO3 Cu(NO3)2 In our example, one copper(II) ion was used up. Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu NO3 NO3 K+ – – – K+ K+ used up + + + Zn 2 NO3 Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 +4 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 The two copper ions that now remain, have a total charge of positive 4. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 –6 Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 And the six nitrate ions have total charge of negative 6. NO3 Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 excess of negative charge Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 So there is an excess of negative charge in the solution in the container on the left. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 NO3 Cu 2 Cu 2 NO3 NO3 excess of negative charge Zn Zn NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 Zn(NO3)2 Now, we’ll focus on the container on the right, with the zinc nitrate solution. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge produced K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 NO3 Cu 2 Cu 2 NO3 NO3 excess of negative charge Zn Zn NO3 Zn 2 Zn(NO3)2 In our example, one zinc 2+ ion was produced… Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + NO3 Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 excess of negative charge Zn +4 NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn Zn NO3 The two zinc 2+ ions that are now present, have total charge of positive 4 A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 Zn Zn NO3 NO3 Cu K+ K+ NO3 Zn 2 Cu 2 Cu 2 NO3 NO3 excess of negative charge Zn –2 NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Cu Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 And the two nitrate ions have a total charge of negative 2. Zn NO3 Zn Zn Zn Zn A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 K+ K+ NO3 Zn 2 NO3 Cu 2 2 NO3 NO3 excess of negative charge Zn Zn NO3 excess of positive charge Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu Zn NO3 Zn NO3 Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 So there is an excess of positive charge in the solution in the container on the right. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu NO3 Cu Cu NO3 salt bridge K+ Zn NO3 K+ K+ NO3 Zn 2 NO3 Cu 2 2 NO3 NO3 excess of negative charge Zn Zn NO3 excess of positive charge Zn Zn Zn Zn Zn Zn Zn Zn NO3 Cu Cu Cu Zn NO3 Zn NO3 Cu Cu NO3 K+ Cu – – – K+ K+ Cu + + + Zn 2 Zn Zn Zn Zn NO3 In order to balance charges, positive ions tend to move (click) toward the left through the salt bridge, away from the side with excess positive charge and toward the side with excess negative charge. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu salt bridge Zn NO3 Cu 2 2 NO3 Zn K+ Zn Cu Cu Cu NO3 NO3 Zn 2 NO3 NO3 excess of negative charge NO3 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 K+ Zn 2 NO3 – – – K+ K+ Cu Cu NO3 NO3 + + + excess of positive charge Zn Zn Zn Zn Zn Zn NO3 And negative ions tend to (click) move toward the container on the right, away from the side with excess negative charge and toward the side with excess positive charge. A – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 NO3 NO3 Cu Cu Cu Cu 2 Cu NO3 K+ Cu 2 NO3 NO3 K+ NO3 Allows ions to move so charges are balanced – – – K+ NO3 Zn salt bridge Cu Cu NO3 + + + Zn K+ NO3 Zn Zn 2 Zn Zn Zn Zn Zn Zn Zn Zn NO3 NO3 Zn 2 Zn Zn Zn Zn Zn Zn NO3 So the salt bridge is an important part of a chemical cell. It allows positive and negative ions to move through it so that the charges in the solutions remain balanced. Without a salt bridge, a chemical cell would not work. V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu Cu Cu 2 NO3 Zn K+ NO3 Zn Zn 2 NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Zn 2 Cu 2 NO3 If we were to replace the ammeter with a voltmeter, Zn Zn NO3 Zn Zn Zn Zn V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Voltage = 1.1 volts –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu Cu Cu 2 Cu 2 NO3 NO3 Zn K+ NO3 Zn Zn 2 NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Zn 2 Zn Zn Zn Zn Zn Zn NO3 Under what are called standard conditions, this cell would initially have a voltage of 1.1 volts. V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu Cu Cu 2 Cu 2 NO3 NO3 Zn K+ NO3 Zn Zn 2 NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Zn 2 Zn Zn Zn Zn Zn Zn NO3 As this cell operates (click), zinc atoms will continue to dissolve to form zinc ions as they lose electrons… V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu Cu Cu 2 Cu 2 NO3 NO3 Zn K+ NO3 Zn Zn 2 NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Zn 2 Zn Zn Zn Zn Zn Zn NO3 And (click) copper 2+ ions will continue to gain electrons as they form copper atoms. V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu Cu Cu 2 NO3 Zn K+ NO3 Zn Zn 2 NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Cu 2 NO3 As zinc atoms on the zinc electrode dissolve, Zn 2 Zinc atoms are dissolved Zn Zn NO3 Zn Zn Zn Zn V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ – + Cu2+ + 2e– Cu Zn Zn2+ + 2e– –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu 2 Cu 2 NO3 NO3 Zn Zn 2 NO3 Cu Cu Zn K+ NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Cu2+ ions are used up Zn 2 Zinc atoms are dissolved Zn Zn Zn Zn Zn Zn NO3 And copper 2+ ions in the copper(II) nitrate solution are used up, the voltage supplied by this cell will gradually decrease, V – + +– +– –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ –+ Voltage = 0 volts –+ K+ K+ NO3 Cu Cu Cu Cu Cu Cu Cu Cu Cu Cu NO3 Zn NO3 Cu 2 Cu 2 NO3 NO3 Zn Zn 2 NO3 Cu Cu Zn K+ NO3 Cu Cu NO3 K+ NO3 – – – K+ salt bridge Cu Cu NO3 + + + Zn Zn Zn Zn Zn Zn Zn Zn NO3 K+ NO3 NO3 Cu2+ ions are used up and will over time, drop to zero. Zn 2 Zinc atoms are dissolved Zn Zn NO3 Zn Zn Zn Zn