PPT - James Madison University
advertisement

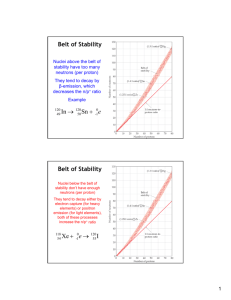
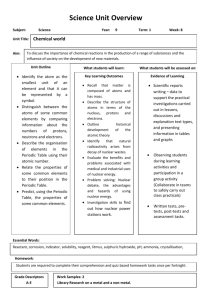
The Nucleosynthesis of Chemical Elements Dr. Adriana Banu, James Madison University January 22, Saturday Morning Physics’11 Big questions We knew for long time that our energy comes from Sun! 1.But what produces it in the Sun? Gravity (which governs planets motion)?! Chemical reactions like on Earth (fuel burning, explosions...)?! In the 1930s we got the answer: nuclear reactions! Namely fusion! What about the other stars?! 2. How were/are the chemical elements created? (nucleosynthesis) The answer is still: nuclear reactions! But which reactions?! How they proceed?! 3. Did nucleosynthesis stop, or continues today? “If in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? Everything is made of atoms.” -Richard Feynman Nobel Prize in Physics, 1965 “If you want to make an apple pie from scratch, you must first create the universe…” “We are star-stuff…” - Carl Sagan • From Aristotle to Mendeleyev In search of the building blocks of the universe… Greek philosophers water 4 building blocks 18th-19th century Lavoisier, Dalton, … air distinction between compounds and pure elements atomic theory revived fire earth 92 building blocks (chemical elements) Periodic Table of Elements 1896 Mendeleyev not to scale Atom = nucleus + electrons -e (10-10 m) +Ze Nucleus = protons + neutrons (10-14 m) • Modern “Alchemy”: radioactivity 1896 Becquerel discovers radioactivity The Nobel Prize in Physics 1903 A. H. Becquerel Pierre Curie Marie Curie emission of radiation from atoms 3 types observed: , and “transmutation” (Helium) • Chart of the Nuclides ~ 3000 currently known nuclides ~ 270 stables only ! ~ 7000 expected to exist Z 118 Sn 50 68 Color Key: Stable + emission - emission particle emission Spontaneous fission N A X Z N A chemical element (X) is uniquely identified by the atomic number Z ! Nuclides that have the same Z but different N are called isotopes ! Mass number: A = N + Z • Nuclear Masses and Binding Energy The binding energy is the energy required to dissasemble a nucleus into protons and neutrons. It is due to the strong nuclear force! M ( Z , N ) = Zm p + Nm n - BE mp = proton mass, mn = neutron mass, m(Z,N) = mass of nucleus with Z protons and N neutrons Thanks to E=mc2, tiny amounts of mass convert into huge energy release… He-4 (2 protons + 2 neutrons) Radium-226 (88 protons + 138 neutrons) Radon-222 (86 protons + 136 neutrons) 1 kg of radium would be converted into 0.999977 kg of radon and alpha particles. The loss in mass is only 0.000023 kg = 23 mg! Energy = mc2 = mass x (speed of light)2 = 0.000023 x (3 x 108)2 = 2.07 x 1012 joules. Equivalent to the energy from over 400 tonnes of TNT!!! 1 kg Ra (nuclear) 4*105 kg TNT (chemical) 238Pu • Modern “Alchemy”:nuclear fusion and fission The process through which a large nucleus is split into smaller nuclei is called fission. Fusion is the opposite! Fission and fusion are a form of elemental transmutation because the resulting fragments are not the same element as the original nuclei. Nuclear fusion occurs naturally in stars ! • Nuclear Reactions A1 Z1 X + Z2 Y A + B A2 A3 Z3 Conservation laws: A4 Z4 A1 + A2 = A3 + A4 (mass numbers) Z1 + Z2 = Z3 + Z4 (atomic numbers) Amount of energy liberated in a nuclear reaction (Q-value): Qval = [(m1 + m2) – (m3 + m4)]c2 initial definition final Qval > 0: exothermic process (release of energy) in stars Qval < 0: endothermic process (absorption of energy) • Stability and Binding Energy Curve Qval >0 fission Qval >0 fusion Qval <0 fusion • What Is the Origin of the Elements? • Nucleosynthesis: the synthesis of Elements through Nuclear Reactions Two original proposals: (full) Big-Bang nucleosynthesis all elements formed from protons and neutrons sequence of n-captures and decays soon after the Big Bang Stellar nucleosynthesis elements synthesised inside the stars nuclear processes well defined stages of stellar evolution Alpher, Bethe & Gamow (“ ”) Burbidge, Burbidge, Fowler & Hoyle (B2FH) Phys. Rev. 73 (1948) 803 Rev. Mod. Phys. 29 (1957) 547 The Nobel Prize in Physics 1967 The Nobel Prize in Physics 1983 Which one is correct? • Big Bang Nucleosynthesis • occurred within the first 3 minutes of the Universe after the primordial quark-gluon plasma froze out to form neutrons and protons • BBN stopped by further expansion and cooling (temperature and density fell below those required for nuclear fusion) • BBN explains correctly the observed mass abundances of 1H (75%), 4He (23%), 2H (0.003%),3He (0.004%), trace amounts (10-10%) of Li and Be, and no other heavy elements Mass stability gap at A=5 and A=8 !!! A=8 BBN No way to bridge the gap through sequence of neutron captures during BB … A=5 After that, very little happened in nucleosynthesis for a long time. temperature and density too small !!! It required galaxy and star formation via gravitation to advance the synthesis of heavier elements. matter coalesces to higher temperature and density… Because in stars the reactions involve mainly charged particles, stellar nucleosynthesis is a slow process. • Stellar life cycle + metals element mixing DEATH explosion Stars thermonuclear reactions Interstellar gas BIRTH gravitational contraction energy production stability against collapse synthesis of “metals” • Hydrogen Burning • slow or fast (explosive) H-burning • almost 95% of all stars spend their lives burning the H in their core (including our Sun). Our Sun is a slow nuclear reactor (a fusion reactor we could not make!) until hydrogen fuel is depleted the life time of our sun depends on the nuclear reaction rates life time of stars depends on their mass: at larger masses burn faster! We are lucky! • Helium Burning: Carbon formation • BBN produced no elements heavier than Li due to the absence of a stable nucleus with 8 nucleons • in stars 12C formation set the stage for the entire nucleosynthesis of heavy elements How is Carbon synthesized in stars? T ~ 6*108 K and ~ 2*105 gcm-3 4He + 4He 8Be 8Be unstable ( ~ 10-16 s) 8Be + 4He 12C • Helium Burning: Oxygen formation • Oxygen production from carbon: + 4He →16O + Carbon consumption ! 12C Reaction rate is very small not all C is burned, but Oxygen production is possible and Carbon-based life became possible… • Nucleosynthesis up to Iron A massive star near the end of its lifetime has “onion ring” structure Carbon burning 12C T ~ 6*108 K ~ 2*105 gcm-3 +12C -> 20Ne + 4He + 4.6 MeV 23Na + 1H + 2.2 MeV Neon burning 9 T ~ 1.2*10 K ~ 4*106 gcm-3 + -> 16O + 4He 20Ne + 4He -> 24Mg + 20Ne Oxygen burning 16O 9 T ~ 1.5*10 K ~ 107 gcm-3 + 16O -> 28Si + 4He + 10 MeV 31P + 1H + 7.7 MeV Silicon burning 9K T ~ 3*10 8 -3 ~ 10 gcm major ash: Fe stars can no longer convert mass into energy via nuclear fusion ! • Nucleosynthesis beyond Iron It was 987,000,000,000,000,000 MILES Away ! 987 QUADRILLION MILES • Abundance of the Elements Almost 5 billion Features: • 12 orders-of-magnitude span • H ~ 75% years ago, our solar system began the • He ~ 23% with its gravitation collapse… • C U ~ 2% (“metals”) journey If we look around us today, we can see what elements were in our interstellar cloud… • Abundance of the Elements Data sources: Earth, Moon, meteorites, stellar (Sun) spectra, cosmic rays... Fe Features: • 12 orders-of-magnitude span • H ~ 75% • He ~ 23% • C U ~ 2% (“metals”) Au Abundance of elements and isotopes are UNIQUE finger prints of various cosmic processes. To interpret and understand them, diverse and vast nuclear physics knowledge is needed!!! Not fully solved! U.S. Nuclear Science [Today and for the Next Decade] General goal: Explain the origin, evolution, and structure of the visible matter of the universe—the matter that makes up stars, planets, and human life itself. The Science – Physics of Nuclei and Nuclear Astrophysics • What is the nature of the nuclear force that binds protons and neutrons into stable nuclei and rare isotopes? • What is the origin of simple patterns in complex nuclei? • What is the nature of neutron stars and dense nuclear matter? • What is the origin of the elements in the cosmos? • What are the nuclear reactions that drive stars and stellar explosions? Nuclear Physics for Astrophysics experiments in laboratory to learn about nuclear reaction in stars Two big problems in nuclear astrophysics: 1. - reactions in stars involve(d) radioactive nuclei use RNB 2. - very small energies and very small cross sections indirect methods Comparison with (reaction) calculations Measurement at lab energies Extract (nuclear structure) information Compare with direct measurements Calculate nuclear reaction probability My research highlights Astrophysical motivation The first sources of light: Population III stars First stars about 400 million yrs. Fate of Massive Pop III Stars G G pp-I,II,III Black Hole Collapse H 3 G dynamic instability Tc 5 107 K crit 0.02 gcm-3 CNO, rp H Explode Interest in M. Wiescher et al., 1989, Ap.J., 343 : 12N(p,)13O Hot pp chains and rap-process chains in low-metallicity objects pp-I: p(p,e+)d(p,)3He(3He,2p)4He pp-II: 7Be(e-,)7Li(p,)4He pp-III: 7Be(p,)8B(+)8Be()4He • process material from pp cycles into CNO nuclei pp-IV: 7Be(p,)8B(p,)9C(+)9B(p)8Be()4He pp-V: 7Be(,)11C(+)11B(p,2)4He rap-I: 7Be(p,)8B(p,)9C(,p)12N(p,)13O(+)13N(p,)14O rap-II: 7Be(,)11C(p,)12N(p,)13O(+)13N)p,)14O rap-III: 7Be(,)11C(p,)12N(+)12C(p,)13N(p,)14O rap-IV: 7Be(,)11C(,p)14N(p,)15O Experimental Setup for 12N(p,)13O study via (12N,13O) proton transfer reaction (Faraday Cup) Er - det . 12 D E - det . (PSSD) 12 N Melamine target C My research @ Madison Radiation Laboratory (JMU) • astrophysical motivation: nucleosynthesis of heavy proton-rich elements beyond iron by photoactivation technique using an electron linear accelerator Measurements of photoneutron reactions induced by activation of a target (stable nucleus), i. e. (,n) reaction rates Medical linac High-resolution Germanium detectors RESOURCES: Existing facilities for research in Nuclear Astrophysics (North America) LENA/TUNL TAMU HIS/TUNL 1. 2. 3. 4. 5. 6. 7. 8. Michigan State University/NSCL Oak Ridge National Laboratory/HRIBF Argonne National Laboratory/ATLAS Lawrence Livermore National Laboratory/NIF TRIUMF/ISAC (Canada) University of Notre Dame/KN Van de Graaf accelerator Yale University/WNSL Florida State University/Super-FN tandem/RESOLUT Overview of main astrophysical processes Present and Future Direction in Physics of Nuclei and Nuclear Astrophysics Rare Isotope Beams Producing radioactive nuclei Protons Neutrons RADIOACTIVE NUCLEI Actually, made by nuclear reactions with stable nuclei: Long lived to very short lived isotopes can be produced and used to produce secondary reactions in laboratory RIB Facilities (Operating or Under Construction) FRIB-facility for rare isotope beams •Ions of all elements from protons to uranium accelerated “Where you could work” (after 2018) What is the origin of the elements in the cosmos? p process FRIB reach Stellar burning Big Bang neutrons NAS report: “Connecting Quarks with the cosmos” 11 questions for the 21st century • how where the elements from iron to uranium made? • In summary • Messages to take away… Nuclear reactions play a crucial role in the Universe: 1. they provide the energy in stars including that of the Sun. 2. they produced all the elements we depend on. 3. nucleosynthesis is on-going process in our galaxy There are ~270 stable nuclei in the Universe. By studying reactions between them we have produced ~3000 more (unstable) nuclei. There are ~4000 more (unstable) nuclei which we know nothing about and which will hold many surprises and applications. Present techniques are unable to produce them in sufficient quantities. It will be the next generation of accelerators and the next generation of scientists (why not some of you?!) which will complete the work of this exciting research field.