Heat of Reaction
advertisement

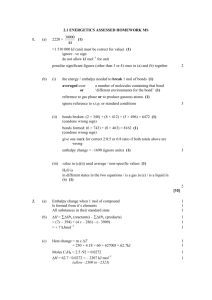
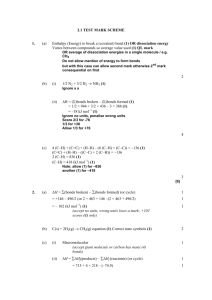
Energy Balance on Reactive Processes Content Introduction Heat of Reaction or Enthalpy of Reaction Relationship Between Enthalpy Change and Heat of Reaction Properties of Heat of Reaction Hess’s Law Heats of Formation Heats of Combustion Energy Balance on Reactive Processes Introduction In any reaction: Energy is required to break the reactant chemical bond Energy is released when product chemical bond is formed Exothermic reaction If energy required to break the reactant chemical bond is less than energy released when product chemical bond is formed the product molecules have lower internal energies than the reactants at the same T and P (i.e ΔH=-ve) Heat of reaction must be released as heat or work to maintain the operation temperature Endothermic reaction If energy required to break the reactant chemical bond is larger than energy released when product chemical bond is formed the product molecules have higher internal energies than the reactants at the same T and P (i.e ΔH=+ve) Energy is need by the process to maintain the operation temperature Heat of Reaction or Enthalpy of Reaction: ΔĤr (T,P) Heat of reaction or enthalpy of reaction - Enthalpy change for a process in which stoichiometric quantities of reactant at T & P reacted completely in single reaction to form a products at the same T & P. - Stoichiometric quantities of reactant means molar amount of the reactant numerically equal to their stoichiometric coefficient. In simple word; Reactants and products: stoichiometric quantities Complete Reaction Reactants are fed at T,P Products are emerging at T,P Hˆ r (T , P) H products H reactants Heat of Reaction : Per mole of what ? 2A + B 3C ΔĤr (100C, 1 atm) =-50 kJ/mol Meaning that: Hˆ r 50kJ 50kJ 50kJ 2 mol A reacted 1 mol B reacted 3 mol C produced If 150 mol C/s is generated, enthalpy change is ΔH= -50 kJ 3 mol C generated 150 mol C generated s = -2500 kJ/s Relationship Between Enthalpy Change and Heat of Reaction Although the Heat of Reaction is defined so, the actual enthalpy change of the reaction depends on how many moles of reactant has been consumed (Extent of reaction). Therefore: Where: vA - stoichiometric coefficient ξ - extent of reaction nA,r - moles of A consumed or generated Hˆ r (T , P) H nA , r vA (n A,out n A,in ) vA H Hˆ r (T , P) nA , r vA Properties of Heat of Reaction 1. Standard heat of reaction (ΔĤr°) - heat of reaction when both reactants and products are at reference conditions (usually 25 C and 1 atm) 2. At low and moderate pressure, ΔĤr is nearly independent of pressure 3. Exothermic (ΔĤr= -ve) and Endothermic (ΔĤr= +ve) 4. ΔĤr depends on how the stoichiometric equation is written CH4 (g) + 2O2(g) CO2(g) + 2H2O(l) ΔĤr1 (25C)= -890.3 kJ/mol for 1 CH4 2CH4 (g) + 4O2(g) 2CO2(g) + 4H2O(l) ΔĤr2 (25C)= -1780.6 kJ/mol for 2 CH4 5. ΔĤr depends on the states of aggregation (gas, liquid, or solid) CH4 (g) + 2O2(g) CO2(g) + 2H2O(l) ΔĤr1 (25C)= -890.3 kJ/mol CH4 (g) + 2O2(g) CO2(g) + 2H2O(g) ΔĤr2 (25C)= -802.3 kJ/mol Internal Energy of Reaction For a reaction takes place in a closed reactor or constant volume ΔÛ r (T) U products U reactant ˆ ˆ U r (T ) H r (T ) RT vi vi gaseous gaseous products reactants Example for the reaction C6H14 (l) + 19/2 O2 (g) 6 CO (g) + 7 H2O (v) Uˆ r (T ) Hˆ r (T ) RT (6 7 19 / 2) Hˆ (T ) 7 / 2 RT r Hess’s Law (Cal’tion of Reaction Heat Normal procedure using calorimeter, however has a limitation If the stoichiometric equation for reaction 1 can be obtained by algebraic operations (multiplication by constant, addition, and subtraction) on stoichiometric equation for reaction 2,3….., then the heat of reaction ΔĤr1 can be obtained by performing the same operations on the heats of reactions ΔĤr2 , ΔĤr3 …. C + 1/2O2(g) CO (incomplete combustion) Alternative method C + O2 CO2 ΔHr1 = -393.51 kJ/mol CO + ½ O2 CO2 ΔHr2 = -282.99 kJ/mol C + ½ O2 (+ ½ O2) H Hˆ 0 r1 CO (+ ½ O2) Hˆ 0 r3 CO2 H Hˆ 0 r 2 Hˆ 0 r 3 Hˆ 0 r1 (Hˆ 0 r 2 ) 393.51 282.99 110.52kJ / mol Class Discussion Example 9.1-1 Example 9.1-2 Example 9.2-1 Heats of Formation Formation reaction – reaction in which the compound is formed from its elemental constituents as they normally occur in nature (e.g. O2 rather than O) standard heat of formation (ΔĤ°f ) - Enthalpy change associated with the formation of 1 mole of compound at a reference temperature (25C) and pressure (1 atm) Standard heat of formation are listed in Table B.1. Standard heat of formation for elemental species (e.g O2) is zero Relationship between standard heat of formation and heat of reaction based on Hess’s Law Hˆ r vi Hˆ fi i ˆ v H i fi products ˆ v H i fi reac tan t Example 9.3-1 Determine the standard heat of reaction for the combustion of liquid n-pentane assuming H2O (l) is a combustion product. Heats of Combustion Standard heat of combustion, heat of combustion of that substance with oxygen to yield specified products (e.g. CO2, H2O) with both reactant and products at 25C and 1 atm. Several value are listed in Table B.1 Relationship between heat of reaction and heat of combustion Hˆ r vi Hˆ ci i ˆ v H i ci reac tan ts ˆ v H i ci products Example 9.4-1 Calculate the standard heat of reaction for the dehydrogenation of ethane C2 H6 C2 H4 + H2 Energy Balance on Reactive Processes Method 1: Heat of Reaction Method preferable when there is a single reaction for which ΔĤ°r is known Reactants ΔH Tout Tin ΔH1 Reactants T=25 oC Products ΔH2 ΔHro Products T=25 oC Method 1: Heat of Reaction Method 1. Complete the material balance 2. Choose reference states for specific enthalpy changes - reactant and products species at 25C and 1 atm for which ΔĤ°r is known - For nonreacting species at any convenient temperature, such as reactor inlet or outlet 3. For a single reaction in a continuous process, calculate the extent of reaction -choose as species A any reactant or product for which the feed and product flow rates are known (n A,out n A,in ) vA 4. Prepare inlet-outlet enthalpy table 5. Calculate each unknown stream component enthalpy for the reactor ; use following eq. 6. Calculate H H Hˆ ro nout Hˆ out nin Hˆ in (single reaction) H Hˆ n j o rj out Hˆ out nin Hˆ in (multiple reactions) reaction 7. Substitute calculated value the required calculations. H in the energy balance equation and complete Energy Balance on Reactive Processes Method 2: Heat of Formation Method preferable when there is a multiple reaction and single reaction for which ΔĤ°r is unknown Reactants ΔH Products Tin Tout ΔH1 ΔH2 Elements 25 oC Method 2: Heat of Formation Method 1. Complete the material balance 2. Choose reference states for specific enthalpy changes - elemental species that constitute the reactants and products in the states in which the elements are found at 25C and 1 atm - For nonreacting species at any convenient temperature 3. Prepare inlet-outlet enthalpy table 4. Calculate each unknown stream component enthalpy 5. Calculate H for the reactor for single or multiple reaction. Note that heat of reaction terms are not required if the element are chosen as references ; use following eq. H nout Hˆ out nin Hˆ in 6. Substitute calculated H value in the energy balance equation and complete the required calculations. Example 9.5-1 The standard heat of reaction for the oxidation of ammonia is given below: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (v) ΔĤ°r=-904.7 kJ/mol 100 mol NH3/s and 200 mol O2/s at 25C are fed into a reactor in which the ammonia is completely consumed. The products gas emerges at 300C. Calculate the rate at which heat must be transferred to or from the reactor, assuming operation at approximately 1 atm. Class Discussion Example 9.5-2 Example 9.5.3 Example 9.5.4 GOOD LUCK FOR YOUR FINAL EXAM