lab report 1 - WordPress.com
advertisement

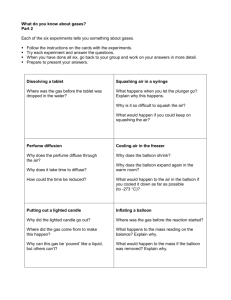
CHEMISTRY I. LAB REPORT TOPIC: Acids and Bases in the Kitchen PREPARED BY: MERVE KASIMOĞULLARI 221 06.04.2012 THE GROUP Merve KASIMOĞULLARI Zeynep GÜNLER Nurefşan EFEOĞLU MERVE KASIMOĞULLARI 06.04.2012 LAB SAFETY RULES 1. Be respectful, follow your teacher's instructions, and don't take shortcuts. 2. Always get your teacher's permission before attempting any laboratory investigation. 3. Read the procedures carefully, paying attention to safety information and cautionary statements. 4. As a responsible citizen, be aware of the location of and the procedure for using the nearest fire alarms, fire-evacuation routes and any other safety equipment, such as fire blankets and eyewash fountains. 5. Never work alone in the laboratory. 6. Walk with care and keep your work area free from all unnecessary clutter. Keep backpacks and other belongings at a certain place designated by your teacher. 7. Dress appropriately on lab day, tie back long hair, remove dangling jewelry and wear closed-toed shoes or sandals in the laboratory. Wear an apron to protect your clothing from staining, burning, or corrosion if instructed by your teacher. 8. Certain products, like hair spray, are flammable and should not be worn while working near an open flame. 9. Wear approved safety goggles when working with or around chemicals, any mechanical device, or any type of flame or heating device. If any substance gets in your eyes or spills or your skin or clothes, rinse it immediately with water and have someone notify your teacher. 10. Wear protective gloves or oven mitts to avoid burns. 11. Be trustworthy and never touch, taste, smell or mix any chemicals unless your teacher instructs you do so. 2 MERVE KASIMOĞULLARI 06.04.2012 12. Use knives and other sharp instruments with extreme care. Always cut an object after placing it on a suitable surface for cutting. 13. Handle plants and animals carefully and wash your hands afterwards. Always treat animals with care and respect. 14. While using electrical equipment, be careful about the wiring, hanging and damaged cords. Be sure your hands are dry and the electrical equipment is turned off before plugging it into the outlet. Turn off all equipment when you are finished using it. 15. Be a responsible and careful scientist by examining glassware to make sure that it is clean and is free of chips and cracks. Report damaged glassware to your teacher. 16. CLEANUP: Clean your work area before leaving! Follow your teacher's directions for washing, unplugging and putting away the equipment. Wash hands with soap and water after working in the laboratory. 17. Stay, work and talk to your own group. Don't leave your group and work area unless instructed by the teacher. Do not sit on counters or tables. PURPOSE The purpose of this experiment is to meet the lab rules, safety procedures and safety equipments. Actually the most important purpose of this experiment is to see the reaction with an acid and a base which are used in the kitchen. HYPOTHESIS When we mix an acid and a base, we will get salt+ water+ gas. 3 MERVE KASIMOĞULLARI 06.04.2012 EQUIPMENT AND MATERIALS We wore the lab coats and glasses for protecting ourselves from all dangerous chemicals. In the lab, water is very useful and after the experiment we cleaned the materials with water. We used acid which is called vinegar and a base which is called carbonate. For mixing them, we have got a plastic flask. We use the balloon to observe the gas which will be occurred by the neutralization. Lastly, we use a candle to see the existence of the gas. EXPERIMENT Our experiment has three parts. 1) We put a balloon which is filled carbonate to the cap of a flask. This flask contains acetic acid in it. And then, we pour the carbonate slowly by using the balloon into the flask which includes 4 % diluted acetic acid. Next we observe that the volume of the balloon is increased because the inside pressure of the balloon is higher than the outside pressure. 2) We coated the balloon with liquid soap which is cleanser and we put a skewer into the top and bottom parts of the balloon not to allow the escaping of the gas. This shows that the balloon is not exploded because of that. 3) We pour the carbon dioxide gas which we obtain during neutralization into the flame of a candle. Carbon dioxide gas extinguishes the flame of the candle. The word and chemical equations for the experiment: Acid + Alkaline 2CH3COOH + CO3 Salt + Water + Gas 2CH3COO + H2O + CO2 4 MERVE KASIMOĞULLARI 06.04.2012 MIXING PROCEDURE First we put the acid into a plastic flask, and then we pour our base into it slowly by a balloon. We mix the chemicals by shaking the bottle. Then we put our balloon at the top of the flask to capture the gas formation. When the experiment was finished, some groups of our class’s balloons were exploded. Actually, when we were doing this experiment we were not only enjoyed but also learned for chemistry. RESULTS After making this experiment which is related to neutralization, we feel that the flask is warmed by the energy, the reaction makes bubble off and our balloon is boomed. CONCLUSION When an acid reacts with a base, the reaction will give water, salt and a gas- commonly carbon dioxide-. This reaction is called NEUTRALIZATION. 5 MERVE KASIMOĞULLARI 06.04.2012 ACIDS AND BASES IN THE KITCHEN REFERENCES: http://science.nsta.org/enewsletter/2003-07/labrules.htm http://kitchenscience.sci-toys.com/acids 6