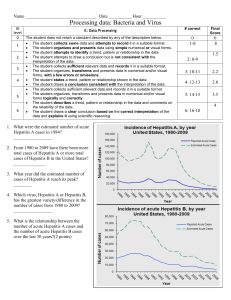
Hepatitis B & C - Health Protection Surveillance Centre
advertisement

Hepatitis C Last updated November 2014 Hepatitis C virus (1) • Virus first identified in 19891 • No vaccine • Routine screening of blood donations in Ireland started in 1991 • Approximately 1,700 people were infected through contaminated blood/blood products prior to this2 • Most new cases of hepatitis C in developed countries like Ireland are in injecting drug users1 • Hepatitis C can also be transmitted sexually and from an infected mother to her baby – but these routes are less common1 • Most cases are initially asymptomatic or mildly symptomatic, but approx. 75% of those infected develop chronic infection3 Hepatitis C virus (2) • Chronic infection can cause cirrhosis, liver cancer (HCC), liver failure and death3 • 5%-20% develop cirrhosis after 20-30 years3 • Of those with cirrhosis, approximately 4% progress to decompensated liver disease annually, 1.5-2.5% develop HCC annually and approximately 80% of those with HCC die annually3 • Disease progression is faster in males, people who were older at infection, those who are co-infected with HIV or HBV and those who consume high levels of alcohol5 • Disease progression is also influenced by metabolic (high BMI, diabetes) and host genetic factors5 Worldwide prevalence hepatitis C infection (source: WHO) Epidemiology of hepatitis C in Ireland • Hepatitis C became notifiable in 2004 • 2004-2013: 12,148 cases notified, peak 2007 (n=1539), significant decrease in recent years – 786 cases notified in 20136 • Notifications include some (but not all) cases diagnosed before 2004 and not previously notified, and some duplicates (full names not always given) • 2012: case definitions altered to explicitly exclude cases known to be resolved (no longer viraemic). Prior to this, laboratory results were frequently insufficient to distinguish chronically infected and resolved cases • 66% of cases notified 2004-2013 were male • Age at notification 2004-2013: median 34 years, mean 36 years • Risk factor data collected 2007-2013 (avail 52%) • 82% of cases with risk factor data were injecting drug users 1800 45 1600 40 1400 35 1200 1000 1400 1119 1539 1506 30 1235 1209 1220 1240 800 25 894 600 786 20 15 400 10 200 5 0 0 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 Year Male Female Unknown sex Mean age males Mean age females Mean age Number of notifications Number of notifications of hepatitis C 2004-2013, by sex and mean age Average annual notification rates per 100,000 Average annual age and sex specific notification rates per 100,000 for hepatitis C, 2010-2013 80 70 60 50 40 30 20 10 0 0-4 5-9 10-14 15-19 20-24 25-34 Age group (years) Male Female 35-44 45-54 55-64 65+ Notification rates of hepatitis C per 100,000 population by HSE area, 2010-2013 Notificaiton rate per 100,000 70 60 50 40 30 20 10 0 E M MW NE NW SE S HSE area 2010 2011 2012 2013 W Most likely risk factor (%) for cases of hepatitis C notified 2010-2013 (where data available, n=2354, 57%) Other and no known risk factor, 5.9% Vertical transmission, 2.7% Possible sexual transmission, 5.7% Blood or blood products, 3.2% Injecting drug user, 82.5% Risk factor trends for cases of hepatitis C notified 2010-2013 Number of notifications 700 Injecting drug user 600 Blood or blood products 500 400 Possible sexual transmission 300 Vertical transmission 200 Other 100 Unknown 0 2010 2011 Year 2012 2013 Hepatitis C laboratory diagnostic data 1989-2004, and estimated prevalence of chronic hepatitis C in Ireland at the end of 2009 • • Hepatitis C testing began in 1989. Approx. 95% of confirmatory HCV testing between 1989 and 2004 carried out by the National Virus Reference Laboratory (NVRL) Study to convert NVRL laboratory information management system (LIMS) specimenbased data into person-based data and estimate prevalence chronic HCV in Ireland at the end of 2009 based on NVRL diagnostic and HPSC notifications data7 Thornton L, Murphy N, Jones L, Connell J, Dooley S, Gavin S, Hunter K, Brennan A. Determination of the burden of hepatitis C virus infection in Ireland. Epidemiol Infect. 2012 Aug;140(8):1461-8 • 10,384 cases of hepatitis C diagnosed by NVRL, 1989-2004 - 55% genotype 1, 4% genotype 2, 39% genotype 3, 1% genotype 1&3, 1 other mixed genotypes and genotypes 4 and 5 - Risk factor available for 76%: 80% were current of former drug users, 16% received blood or blood products in the past • Estimated national prevalence chronic hepatitis C end 2009: 0.5-1.2% (20,000-50,000) • More recent estimates of levels of underdiagnosis8 indicate that prevalence in Ireland is more likely to be 0.5-0.7% (20,000-30,000) Studies of hepatitis C prevalence in Ireland Injecting drug users • Studies of injecting drug users (mostly heroin) in Ireland, between 1992 and 2006: hepatitis C antibody (anti-HCV) prevalence in this population 52-84%9-17 • 2011 prison study found that 54% of prisoners with a history of injecting heroin were anti-HCV positive and 41.5% of prisoners with a history of injecting any drugs were anti-HCV positive18 Antenatal females: Universal HCV screening studies in 2 large maternity hospitals in Dublin: 0.7% & 0.9% anti-HCV positive19,20 New blood donors: Irish Blood Transfusion Service: 0.02% of new donors tested 1997 to 2012 were anti-HCV positive (personal communication: IBTS) Asylum seekers: Balseskin reception centre: 1% of those tested, under the voluntary health screening programme, between 2004 and 2012, were positive for chronic HCV infection21 Hepatitis C anti-viral treatment • Anti-viral treatments are available for hepatitis C • Goal of treatment is to eradicate the virus from the patient’s blood and prevent long-term liver damage and death • Treatment success is measured by SVR12 or SVR24 – sustained virological response – undetectable RNA at 12 or 24 weeks after completion of treatment • SVR is associated with a reduction in liver-related mortality,22,23 hepatocellular carcinoma,22,23 hepatic decompensation22, liver tranplantation23 and all-cause mortality23 Treatment standard of care in Ireland prior to 2012 • Pegylated interferon (Peg-IFN) & ribavirin (RBV)24 – 24 weeks for genotype 2 or 3 HCV SVR: >75% – 48 weeks for genotype 1 HCV SVR: 40-50% • Significant side effects - mainly from IFN24 • Factors associated with better response to treatment – Genotype 2/3 rather than genotype 125,26 – Younger age at treatment26 – Lower viral load at treatment25, 26 – Disease stage: non-cirrhotic25 – host genetics: IL28B CC genotype, rather than TT26 New treatments currently available in Ireland: Direct acting antivirals (DAA) • In 2012 two protease inhibitors (Boceprevir (Boc) and Telaprevir (Tel) were recommended by NCPE for reimbursement in Ireland. These were for use in patients with Genotype 1 hepatitis C infection in combination with Peg-IFN and RBV • Response guided treatment – clear stopping rules – based on viral load • Additional side effects –rash, haematological, gastrointestinal • SVR in genotype 1 patients –improvement on previous treatments - Boceprevir + Peg-IFN + RBN: 54-75%27 - Telaprevir + Peg-IFN + RBN: 61-75%27 Newer Direct Acting Antivirals • Many new therapies in development and undergoing licensing and reimbursement decisions • Various combination therapies using new direct acting antivirals, with and without RBV, and with and without Peg-IFN (all-oral treatment possible) • Achieving high SVRs in clinical trials, even among patients with cirrhosis and those who have previously failed treatment • Daclatasvir, sofosbuvir and simeprevir are currently licensed in Europe and reimbursement recommendations in respect of these drugs are under review by the National Centre for Pharmacoeconomics (NCPE) www.ncpe.ie References (1) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. World Health Organization: Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva; April 2014 Health Protection Surveillance Centre. National Hepatitis C database. 2012 Report. Available at: http://www.hpsc.ie/AZ/Hepatitis/HepatitisC/HepatitisCDatabase/BaselineandFollow-upReports/ Global Burden of Hepatitis C Working Group: Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol 2004, 44:20-29. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST: Global epidemiology of hepatitis C virus infection: new estimates of agespecific antibody to HCV seroprevalence. Hepatology 2013, 57(4):1333-1342 Seeff LB. The history of the "natural history" of hepatitis C (1968-2009). Liver Int. 2009 Jan;29 Suppl 1:89-99. Health Protection Surveillance Centre. HPSC annual report 2013 Thornton L, Murphy N, Jones L, Connell J, Dooley S, Gavin S, Hunter K, Brennan A. Determination of the burden of hepatitis C virus infection in Ireland. Epidemiol Infect. 2012 Aug;140(8):1461-8 Public Health England. Hepatitis C in the UK: 2013 report. Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317139502302 Smyth R, Keenan E, Dorman A, O’Connor J: Hepatitis C infection among injecting drug users attending the National Drug Treatment Centre. Ir J Med Sci 1995, 164(4):267-268 Smyth BP, Keenan E, O’Connor JJ: Bloodborne viral infection in Irish injecting drug users. Addiction 1998, 93(11):1649-1656. Smyth BP, Keenan E, O’Connor JJ: Evaluation of the impact of Dublin’s expanded harm reduction programme on prevalence of hepatitis C among short-term injecting drug users. J Epidemiol Community Health 1999, 53:434-435. Cullen W, Bury G, Barry J and O’Kelly F (2000) Drug users attending general practice in the Eastern Regional Health Authority area. IMJ, 93(7): 214–217 Grogan L, Tiernan M, Geoghegan N, Smyth BP, Keenan E: Bloodborne virus infections among drug users in Ireland: a retrospective cross-sectional survey of screening, prevalence, incidence and hepatitis B immunisation uptake. Ir J Med Sci 2005, 174(2):14-20. Cullen W, Bury G, Barry J, O’Kelly FD: Hepatitis C infection among drug users attending general practice. Ir J Med Sci 2003, 172(3):12327 References (2) 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. Cullen W, Stanley J, Langton D, Kelly Y, Bury G: Management of hepatitis C among drug users attending general practice in Ireland: Baseline data from the Dublin area hepatitis C in general practice initiative. Eur J Gen Pract 2007, 13:5-12 Long, Jean (2006) Blood-borne viral infections among injecting drug users in Ireland, 1995 to 2005. Overview 4. Health Research Board, Dublin Allwright S, Bradley F, Long J, Barry J, Thornton L and Parry JV (2000) Prevalence of antibodies to hepatitis B, hepatitis C and HIV and risk factors in Irish prisoners: results of a national cross-sectional survey. BMJ, 321: 78–82 Drummond A, Codd M, Donnelly N, McCausland D, Mehegan J, Daly L, Kelleher C: Study on the prevalence of drug use, including intravenous drug use, and blood-borne viruses among the Irish prisoner population. Dublin: National Advisory Committee on Drugs and Alcohol; 2014 Martyn F, Phelan O, O'Connell M. Hepatitis C: is there a case for universal screening in pregnancy? Ir Med J. 2011 May;104(5):144-6 Lambert J, Jackson V, Coulter-Smith S, Brennan M, Geary M, Kelleher TB, O'Reilly M, Grundy K, Sammon N, Cafferkey M. Universal antenatal screening for hepatitis C. Ir Med J. 2013 May;106(5):136-9 Brennan M, Boyle PJ, O'Brien AM, Murphy K. Health of Asylum seekers - are we doing enough? ICGP Forum magazine, November 2013 Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011 Nov;9(11):923-30 Van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012 Dec 26;308(24):2584-93 Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006 Sep;55(9):1350-9 Asselah T, Estrabaud E, Bieche I, Lapalus M, De Muynck S, Vidaud M et al. Hepatitis C: viral and host factors associated with nonresponse to pegylated interferon plus ribavirin. Liver Int. 2010 Oct;30(9):1259-69 Chuang WL, Yu ML. Host factors determining the efficacy of hepatitis C treatment. J Gastroenterol. 2013 Jan;48(1):22-30 Ramachandran P, Fraser A, Agarwal K, Austin A, Brown A, Foster GR et al. UK consensus guidelines for the use of the protease inhibitors boceprevir and telaprevir in genotype 1 chronic hepatitis C infected patients. Aliment Pharmacol Ther. 2012 Mar;35(6):64762