Chapter 08
advertisement

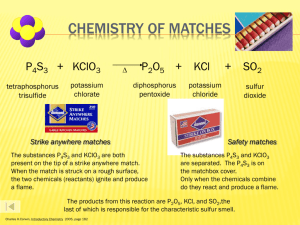
Chapter 8: Chemical Reactions OBJECTIVES 1. Identify when a chemical reaction occurs 2. Write chemical equations 3. Balance chemical equations 4. Predict the products of reactions when the reactants are known 5. Use the Activity series to determine if a reaction will occur A. Energy and Chemical Reactions _____________ EXOTHERMIC Releases Energy such as heat, sound, and light. Feels Hot. ENDOTHERMIC _____________ Absorbs Energy such as heat; feels cold. A. Energy and Chemical Reactions ACTIVATION ENERGY Energy needed to trigger a reaction. EXO ENDO B. Indications of chemical reactions Evolution of heat and light Burning Alcohol Formation of a gas NaOCl + HCl NaCl + H20 + Cl2 Formation of a precipitate CuSO4 + NaOH Cu(OH)2 + Na2SO4 Color change HNO3 + Cu Cu(NO3)2 + NO2 + H2O C. Chemical Equations Right side are PRODUCTS Left side are REACTANTS PbCl2 + Na2CrO4 PbCrO4 Yields Number of moles or particles + 2NaCl C. Chemical Equations THE MAGNIFICENT SEVEN These elements exist in nature as diatomic molecules, never as a lone atom. H2, N2, O2 F2, Cl2, Br2, I2 And Hydrogen! C. Chemical Equations - Symbols (s) or ↓as in NaCl (s) (l) as in H2O (l) (g) as in H2O (g) (aq) as in NaCl (aq) 75o C or Pd Yields Reversible reaction Solid or precipitate Liquid gas Aqueous i.e. dissolved in water Heat added Catalyst used D. Balancing Equations During a process called electrolysis (electric current) water is converted to hydrogen gas and oxygen gas. NEVER EVER CHANGE A SUBSCRIPT 2 H2O 2 H2 4 2 H 2 4 2 1 O 2 + O2 D. Balancing Equations Fe2O3 Treat Polyatomic ions as one unit + 3 H2SO4 Fe2(SO4)3 + 3H2O 2 Fe 2 3 O 1 3 6 2 H 2 6 3 1 SO4 3 E. Types of reactions 1. Synthesis Reactions General Form: A + B AB Definition: Two substances forming a new compound Mg Fe + + O2 O2 Assuming Fe+4 Assuming Fe+3 Fe + O2 E. Types of reactions 2. Decomposition Reactions General Form: AB A + B Definition: A compound breaks down into 2 or more H2O HgO 𝐸𝑙𝑒𝑐𝑡𝑟𝑖𝑐𝑖𝑡𝑦 Δ Metal hydroxides Metal oxides and water Ca(OH)2 Al2(CO3)3 Metal carbonates Metal oxides and CO2 E. Types of reactions 3. Single Replacement Reactions General Form: A + BC B + AC OR Y + AX X + AY Definition: A metal or halogen switches with another Al + Pb(NO3)2 Na + H2O F2+ HCl E. Types of reactions 4. Double Replacement Reactions General Form: AX + BY BX + AY Definition: Two metals swap positions KI FeS + + Pb(NO3)2 HCl E. Types of reactions 5. Acid Base Reactions (Really a Double replacement) General Form: HX + BOH BX + H2O Definition: Double replacement that makes water Acids start with H Bases end with OH HCl + NaOH E. Types of reactions 6. Combustion Reactions General Form: CxHyOz + O2 CO2 + H2O Definition: Hydrocarbon burns making CO2 and H2O CO2 and H2O are ALWAYS the products O2 indicates combustion C3H6O2 C8H18 + + O2 O2 F. Factors that influence Reaction rate Reaction rate is determined by particle collisions Successful collisions: Collide with each other Have the correct orientation Have enough kinetic energy to break bonds Na Cl Na Cl K K F. Factors that influence Reaction rate K Na Cl F. Factors that influence Reaction rate Four major influences: Temperature Increase speeds up reactions Faster particles (more KE) Concentration Higher concentration speeds up reactions More chances to collide Surface area Higher SA speeds up reactions More chances to collide Catalyst F. Iodine Clock Reaction G. Activity Series Most Reactive Li Rb K React with cold water and acids, replacing hydrogen. React with oxygen forming oxides. F2 Cl2 Ba Br2 Sr I2 Ca Na Mg Al Mn Zn React with STEAM and acids replacing hydrogen. React with oxygen forming oxides. Cr Fe Cd Co Ni Sn Pb H2 Sb Bi Do NOT react with water. React with acids replacing hydrogen. React with oxygen forming oxides. React with oxygen forming oxides. Cu Hg Least Reactive TABLE OF HALOGENS Ag Pt Au Fairly unreactive. H. Solubility Chart