Chapter 3 Practice Test
advertisement
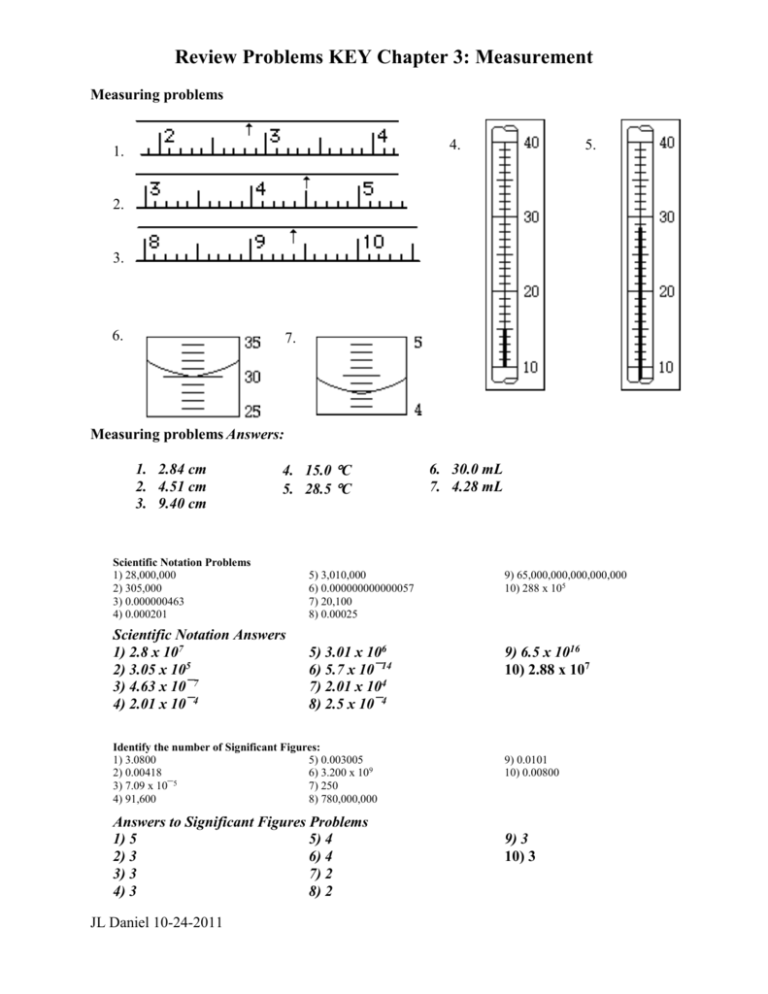
Review Problems KEY Chapter 3: Measurement Measuring problems 4. 1. 5. 2. 3. 6. 7. Measuring problems Answers: 1. 2.84 cm 2. 4.51 cm 3. 9.40 cm 4. 15.0 C 5. 28.5 C 6. 30.0 mL 7. 4.28 mL Scientific Notation Problems 1) 28,000,000 2) 305,000 3) 0.000000463 4) 0.000201 5) 3,010,000 6) 0.000000000000057 7) 20,100 8) 0.00025 9) 65,000,000,000,000,000 10) 288 x 105 Scientific Notation Answers 1) 2.8 x 107 2) 3.05 x 105 3) 4.63 x 10¯7 4) 2.01 x 10¯4 5) 3.01 x 106 6) 5.7 x 10¯14 7) 2.01 x 104 8) 2.5 x 10¯4 9) 6.5 x 1016 10) 2.88 x 107 Identify the number of Significant Figures: 1) 3.0800 5) 0.003005 2) 0.00418 6) 3.200 x 109 3) 7.09 x 10¯5 7) 250 4) 91,600 8) 780,000,000 Answers to Significant Figures Problems 1) 5 5) 4 2) 3 6) 4 3) 3 7) 2 4) 3 8) 2 JL Daniel 10-24-2011 9) 0.0101 10) 0.00800 9) 3 10) 3 Math with Significant Figures (addition/subtraction): 1) 3.461728 + 14.91 + 0.980001 + 5.2631 2) 23.1 + 4.77 + 125.39 + 3.581 3) (2.71 x 106) - (5.00 x 104) 4) 0.04216 - 0.0004134 5) 564,321 - 264,321 Answers to math with significant figures (addition/subtraction): 1) 24.61 3) 2,660,000 5) 300,000. or 2) 156.8 4) 0.04175 3.00000 x 105 Math with significant figures (multiplication/division): 1) (3.4617 x 107) ÷ (5.61 x 10¯4) 2) (2.68 x 10¯5) x (4.40 x 10¯8) 3) (4.7620 x 10¯15) ÷ [(3.8529 x 1012) (2.813 x 10¯7) (9.50)] 4) (8.41 x 106) x (5.02 x 1012) Answers to math with significant figures (multiplication/division): 1) 6.17 x 1010 3) 4.63 x 10-22 -12 2) 1.18 x 10 4) 4.22 x 1019 Metric system problems: I. Given either the name or the symbol of the prefix, give the other: 1) 2) c k 4) nano II. Given the prefix size, give its name: 8) 10-12 9) 1,000 10) 10-1 Metric System answers: 1) centi2) kilo3) micro4) n Convert the following metric measures: 1. 0.75 kg to milligrams 2. 1500 millimeters to cm 3. 0.02390 g to µg 4. 52 pm to nanometers 5. 65 ng to cg 1. 750 000 mg 2. 150 cm 3. 23900 µg 4. 0.052 nm 5. 0.0000065 cg or 6.5 x 10-6 c JL Daniel 10-24-2011 5) 6) 7) milli d pico 11) 10-2 12) 0.000001 5) m 6) d 7) p 8) pico- 9) kilo10) deci11) centi12) micro- Density problems: 1) A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density? 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. 3) What is the mass of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL. 4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? 5) A flask that weighs 345.8 g is filled with 225 mL of carbon tetrachloride. The mass of the flask and carbon tetrachloride is found to be 703.55 g. From this information, calculate the density of carbon tetrachloride. Answers To Density Problems 1) 2.70 g/mL 2) 13.6 g/mL 3) 158 g 4) 8.9 g/cm3 5) 1.59 g/mL JL Daniel 10-24-2011 JL Daniel 10-24-2011










