Lesson 1 - Alcohols
advertisement

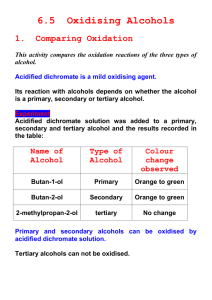
Key area: Oxidation of foods Overview In this section, learn how oxidation reactions in foods convert alcohols to aldehydes and ketones, and study the role of antioxidants in the preservation of foods. . a) Oxidation of alcohols Learning intention In this section, learn about the products of oxidation of primary, secondary and tertiary alcohols. Find out about important mild oxidising agents and learn how to spot an oxidation reaction in a carbon compound. After completing this lesson you should be able to : Branched-chain alcohols, with no more than eight carbon atoms in their longest chain: can be named from structural formulae structural formulae can be drawn from names molecular formulae can be written from names Draw the structural formula for, and name isomeric alcohols, including primary secondary and tertiary alcohols where appropriate. Hydrogen bonding can be used to explain the properties of alcohols including, boiling points, melting points, viscosity and solubility/miscibility in water. Structures of diols and triols and the effect of hydrogen bonding on properties, including, boiling points, melting points, viscosity and solubility/miscibility in water, of these molecules. In the laboratory, hot copper(II) oxide or acidified dichromate(VI) solutions can be used to oxidise primary and secondary alcohols: Primary alcohols are oxidised, first to aldehydes and then to carboxylic acids. Secondary alcohols are oxidised to ketones Tertiary alcohols cannot be oxidised When applied to carbon compounds, oxidation results in an increase in the oxygen to hydrogen ratio and reduction results in a decrease in the oxygen to hydrogen ratio. Introducing alcohol This page explains what alcohols are, and what the difference is between primary, secondary and tertiary alcohols. Naming alcohols, aldehydes and ketones Information from BBC Bitesize on the rules for naming organic compounds. Alkanals COPY Summary of Aldehyde Nomenclature rules 1.Aldehydes take their name from their parent alkane chains. The -e is removed from the end and is replaced with -al. COPY Akanones COPY Summary of Ketone Nomenclature rules 1) Ketones take their name from their parent alkane chains. The ending -e is removed and replaced with -one. COPY Classification of alcohols H H H C C H OH CH3 H H C CH3 O H H3C C CH3 H H H H C C C H OH H propan-2-ol O H CH3 Secondary alcohol, TWO C’s joined to the C bonded to the OH group Primary alcohol, ONE C joined to the C bonded to the OH group COPY Tertiary alcohol, THREE C’s joined to the C bonded to the OH group CH3 H H3C C CH3 OH 2-methylpropan-2-ol COPY COPY Oxidation reactions The most important oxidation reactions are oxidation of alcohols (alkanols) and aldehydes (alkanals), using a variety of oxidising agents. Oxidation just means joining with oxygen. Complete combustion is an extreme oxidation reaction. Example: complete combustion of methanol 2 CH3OH + 3 O2 → 2 CO2 + 4 H2O Alcohols burn in oxygen to produce carbon dioxide and water. In organic chemistry, oxidation can mean either adding oxygen or removing hydrogen. The oxidations to remember are: 1.primary alcohol →aldehyde →carboxylic acid; 2.secondary alcohol→ketone →no further oxidation; 3.tertiary alcohol →not oxidised. COPY Oxidation of Alcohols Primary alcohols can be oxidised by a number of oxidising agents, in two stages, 1st Stage - Hydrogen is lost; 2nd Stage - oxygen is gained. When applied to carbon compounds, oxidation results in an increase in the oxygen to hydrogen ratio. H 1st R C O oxidation H + O R O R C H + H2O aldehyde O H 2nd C H aldehyde + O oxidation O R C O H Carboxylic acid Secondary alcohols can be oxidised to form ketones, Tertiary alcohols do not undergo oxidation. O R C ketone R1 Experiment The oxidising agents COPY It is important to remember the colour changes which occur in the reactions, from starting to final colours. Oxidising agents must always be reduced. COPY Reaction sequence involving primary and secondary alcohols H hydration H C C C H oxiation O H - 2H H H propan-1-ol H H H H + H2O H H C C H H C C H H H H O alkanal hydration H H C H H C O oxidation H C H H H H propan-2-ol secondary alcohol - 2H H C H H H C C H H alkanoic acid H + H2O +O H propanoic acid H propene oxiation C propanal primary alcohol C H H C C O propanone alkanone H O C O H Higher Chemistry Unit 2 – Nature’s Chemistry Alcohols Multiple Choice Questions 1. Which of the following structural formulae represents a tertiary alcohol? Answer B. 2. Ethanol is prepared from glucose by the process of A. respiration B. fermentation C. catalytic hydration D. photosynthesis Answer B. 3. Which alkanol can, on dehydration, produce a pair of isomeric alkenes? A. propan-2-ol B. pentan-3-ol C. hexan-3-ol D. heptan-4-ol Answer C. 4. Which product(s) would be expected upon dehydration of the following alcohol? A. 2-methylbut-2-ene only B. 2-methylbut-1-ene and 2 methylbut-2-ene C. 2-methylbut-1-ene only D. 2-methylbut-1-ene and 3-methylbut-1-ene Answer B. 5. What type of reaction takes place when propene is formed from propanol? A. condensation B. hydrolysis C. dehydration D. hydration Answer C. 6. When an alcohol is completely oxidised the products are A. carbon dioxide and hydrogen B. carbon dioxide and water C. carbon monoxide and hydrogen D. carbon dioxide and water Answer B. 7. When acidified potassium dichromate is used to oxidise an alcohol the colour changes from A. blue to red/orange B. black to brown C. green to orange D. orange to green Answer D. 8. The type(s) of alcohol which can be oxidised are A. primary only B. secondary only C. tertiary only D. none of the above answers Answer D. 9. 2-methyl hexan-1-ol and 3-methyl hexan-1-ol are A. isomers which are both primary alcohols B. isomers which are both primary alcohols C. not isomers but both primary alcohols D. not isomers but both secondary alcohols Answer A. 10.2-methyl pentan-2-ol A. is a primary alcohol and can be oxidised B. is a secondary alcohol and can be oxidised C. is a tertiary alcohol and can be oxidised D. is a tertiary alcohol and cannot be oxidised Answer D.