May - York College of Pennsylvania
advertisement

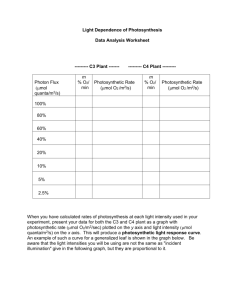
Introduction Can Association Patterns Between Unrelated Stimuli Classical Conditioning is a type of learning in which an organism H0: There will be no learned association in the experimental group, and therefore, no difference from the control groups. Materials and Methods General Setup The pea plants were grown in 6 in. pots in potting soil with perilite fertilizer. Growing took place in a greenhouse under optimal growing lights that were set up on a timer to run from 6 a.m. to 9 p.m. Three groups were established with 18 plants in each group. A structure was built over each group with hanging wires for the tendrils of the pea plants to coil around (See Figure 1). The groups were separated from each other with a thin board that blocked light and wind between groups. Fans were set up in front of control group 2 and the experimental group to induce winds of approximately 6-7 mph. The experimental group was subject to the wind from a fan at night. At 9 p.m. every night the fan turned on and, within two minutes, the lights in the entire greenhouse turned off. The following morning at 5 a.m., the fan turned off, and within two minutes, the lights turned on. Control group 1 grew in the greenhouse at the same time and was subject to the exact same environment, however, it received no wind from fans. Control group 2 received wind from a fan for the same duration of time that the experimental group did. The fan, however, was not in conjunction with the lights. It turned on at 10 p.m. and off the next morning at 6 a.m. The experiment began 14 days after planting and lasted 16 days. Fig. 2 There was great individual variance in the photosynthetic rates of each plant, as well as, great variance in the net change once the wind was applied. No significant differences were found between the groups. The photosynthesis results can be found in Figures 3-5. Morphological results can be found in Table 1. Table 1. ANOVA Statistical results of morphological comparisons between each group. CG1 vs. CG2 CG1 vs. EG CG2 vs. EG Photosynthetic Study Fig. 3 Photosynthetic Rate 16 Time 1 Time 2 12 µmol CO2/cm2/s The eight healthiest plants were chosen from each group to be tested. An infrared gas analyzer* (IRGA) was clamped on a leaf that was still attached to the plant. The photosynthetic rate was allowed four minutes to stabilize and then a reading was taken. The fan was then turned on for an additional three minutes and another reading was taken. The leaf inside the chamber that was being measured was not subject to the wind. It was hoped that the plant would have a systemic response to the wind and photosynthesis across the entire plant would slow. 8 4 1 Morphological Study 2 3 4 5 6 7 8 Plants The small leaves of pea plants did not fill the chamber of the IRGA. Scion** was used to compute the exact area Fig. 4 Photosynthetic Rate Changes in Control Group 2 of each leaf. This information was then entered back into 16 the IRGA so that photosynthetic rate could be accurately Time 1 determined per cm2. Stomatal peels were taken from two leaves per plant. This involved brushing clear nail polish onto the leaves. Once dry, the nail polish was peeled off the leaf and mounted onto a microscope slide (Coombs et al. 1985). The stomata of the leaves were counted to determine stomatal density on top and bottom of the leaves. Time 2 12 Biomass of the plants was measured. Dry weights of roots, stems, and leaves were taken individually. Roots were washed and patted dry to remove dirt. The leaves, stems, and roots were dried in a drying oven at 28oC for 4 Fig. 5 days. They were then massed on a balance. Specific weights of leaves were compared between groups as well. Two leaves were taken from each plant and the areas were determined using scion** as mentioned before. The leaves were then dried in the oven and weighed. The mass per unit area of the leaves was an indication of the thickness of the leaves. Average internode length was determined by dividing the entire length of the above ground portion of the plant by the number of internodes present. Stomatal Ratio (T/B) * p=0.0264 ns ns Stomatal Density- Top * p=0.0113 ns ns Stomatal Density- Bot. * p<0.05 ** p=0.0016 ns Internode Length * p<0.05 * p<0.05 ns Biomass- Leaf ns ns ns Biomass- Stem ns ns ns Biomass- Root ns ns ns Leaf Sp. Weight ns * p<0.05 ** p<0.01 Photosynthetic Rate Changes in Control Group 1 µmol CO2/cm2/s H1: It is proposed that by the end of the experiment, when the fans are turned on, the photosynthetic rate will drop in full sunlight because the plants anticipate the lights being turned off. Fig. 1 8 Discussion The photosynthetic rates were too individual to determine any trends. The plants were in the exact same environment during the experiment and during the testing. It is difficult to determine why one plant would photosynthesize at a rate of under 4 and another at a rate of over 15. There are a myriad of possible reasons including genetic variability and equipment/operator error. With this great range of photosynthesis, it is not surprising that the time 2 results for each plant were just as scattered. There were no clear results from the photosynthesis testing. Therefore, we must fail to reject the null hypothesis. There was no significant difference between the experimental and control groups. In conclusion, the plants were not able to associate the two unrelated stimuli, wind and light. The photosynthetic rates did not plummet when the fans were turned on. However, the plants did respond to the wind by altering their morphology. Table 1 shows that the plants that received wind were morphologically different than those that did not receive wind. This is evidence that the pea plants do have the apparatus to perceive and respond to wind. Literature Cited 4 1 2 3 4 5 6 7 8 Plants Photosynthetic Rate Changes in the Experimental Group Time 1 16 Time 2 12 µmol CO2/cm2/s The purpose of this research was to determine if an association pattern between light and the mechanical stimulus wind exists in the common pea plant Pisum sativum L. Stated simply, the goal is to entrain the plant to perceive the wind as the cause of the lights going off. If this is accomplished, by the end of the training period, the wind should cause a severe drop in the photosynthetic rate due to anticipation of the lights going off. Brandon May, and Bruce Smith Department of Biological Sciences, York College of Pennsylvania Photosynthetic Rate Plants and animals share a common cryptochrome, or blue light receptor, that is responsible for a variety of light responses including entrainment of circadian rhythms. It is proposed that this cryptochrome could pre-date the divergence of plants and animals from a common ancestor (Cashmore et al. 1999). Pea plants are also sensitive to mechanical stimulations, such as vibrations when the wind blows and touch (Galston and Jaffee 1966:68). Wind has been shown to increase pest resistance in the common bean (Cipollini 1997). Therefore, it is evident that pea plants have the ability to perceive and respond to both light and wind. be Instilled in the Plant Pisum sativum L.? Photosynthetic Rate is entrained to associate two unrelated external stimuli (Myers 2001). The classic example is the Pavlovian dog salivating when the dinner bell rang because it associated the ring with the introduction of food. It anticipated the presentation of food, when the bell rang. Dr. James McConnell and associates performed similar studies on the planarian, Dugesia dorotocephala (Thompson 1955). These flat worms have the ability to perceive and respond to light and shock stimuli. When the intensity of the light increased, the animals were shocked. This caused a longitudinal contraction of the body. At the end of the experiment, only changing the intensity of the light caused the contraction, as if shock was anticipated. Cross sections of regenerated planarians retained the entrained association; the head sections that grew new tails and the tail sections that grew new heads both responded as significantly as intact planarians (Jacobson and McConnell 1959). The fact that the tail sections retained the association leads to a possible biochemical basis for learning. Results Cashmore, A.R., Jarillo J.A., Liu D., and Wu Y.J. 1999. Cryptochromes: Blue Light Receptors for Plants and Animals. Science 284 760-5. Cipollini, D.F. 1997, Wind-induced mechanical stimulation increases pest resistance in common bean: Oecologia. 111 84-90. Coombs, J., Hall, D.O., Long, S.P. and Scurlock, J.M.O. 1985. Techniques in Bioproductivity and Photosynthesis. 2nd ed. Pergamon Press, New York, NY. Galston A.W. and Jaffe M.J. 1966. Physiological Studies on Pea Tendrils. I. Growth and Coiling Following Mechanical Stimulation. Plant Physiology 41 1014-1025. Galston A.W. and Jaffe M.J. 1968. The Physiology of Tendrils. Annual Review of Plant Physiology 19 417-434. Jacobson, A.L. and McConnell, J.V. 1959. The effects of Regeneration Upon Retention of a Conditioned Response in the Planarian. Journal of Comparative & Physiological Psychology 52 1-5. Thompson, R. and McConnell, J.V. 1955. Classical Conditioning in the Planarian, Dugesia dorotocephala. Journal of Comparative & Physiological Psychology 48 65-68. 8 Acknowledgements I acknowledge the following people for their contributions to the project: Dr. Bruce Smith, mentor; Dr. Karl Kleiner; and Dr. Bradley Rehnberg 4 1 2 3 4 5 Plants 6 7 8 *LI-COR Li6400 Infrared gas analyser. LICOR Bioscience, Inc. Lincoln, NE **Scion Image Grabber Software. Scion Corp. Frederick, MD