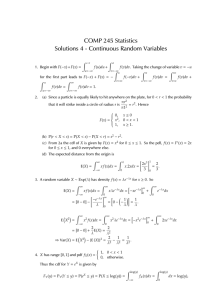
is:rate = k[NO 2 ]
advertisement
![is:rate = k[NO 2 ]](http://s3.studylib.net/store/data/009236352_1-79e35dd115c5ac386e1d8834f2c7d21b-768x994.png)
Kinetics: Rates and Mechanisms of Reactions Chemical Kinetics tells us: …how fast a reaction will occur …how molecules react (MECHANISM) a mechanism is a sequence of steps that lead to the product 1 Factors Affecting the rate: 1. concentration: 2. temperature: generally a 10oC increase will double the rate 3. nature of the reactant: i.e. surface area 4. catalyst: (two types: homogenous and heterogeneous) 5. mechanism: (orientation, shape, & order) COLLISION THEORY = CAPPING A MARKER 2 Rate of RXN = The increase in concentration of a product per unit time. or The decrease in concentration of a reactant per unit time. 3 Conc. is usually measured in M (Molarity= mol/L) for solutions. Rate = M time = mol L•s or mol•L-1•s-1 or M•s -1 Square brackets [ ] are often used to express molarity (i.e. [HCl] means Molarity of HCl) Since many reactions involve gases, P is often used for concentration. n n from PV nRT, P RT and has units of ... V V moles/L 4 Consider the reaction (net ionic eq.): 3ClO- (aq) 2Cl-(aq) + ClO3-(aq) Rate could be defined in at least 3 ways: (3 coefficients and ions) 1. appearance of ClO3- [ClO 3 ] rate time 2. appearance of Cl- [Cl - ] rate time 3. disappearance of ClO- Disappearance [ClO - ] rate time 5 Consider the reaction (net ionic): 3ClO- (aq) 2Cl-(aq) + ClO3-(aq) 1. appearance of ClO3- [ClO 3 ] rate time 2. appearance of Cl- [Cl - ] rate time 3. disappearance of ClO- Disappearance [ClO - ] rate time Question: Are these three rates equal? 6 Consider the reaction (net ionic): 3ClO- (aq) 2Cl-(aq) + ClO3-(aq) Let’s make these three rates equal. [ClO -3 ] [Cl - ] [ClO - ] rate time 2time 3time Note the use of coefficients and the sign 7 General Form: aA + bB cC + dD rate = [C] ctime = [D] = dtime “PRODUCTS” - [A] atime =- [B] btime “REACTANTS” 8 conc rate time slope Average rate = slope (over time period) 0.30- 0.74 0.0146 40 - 10 - 7 4 1 0. 0 6 2. 0 -0 7. 0 ep o l s 01 - 04 negative sign 9 conc rate time instantaneous rate = tangent slope (changing) WHY? Collision Theory! 10 y Slope tangentat pointof interest x 11 IF we can now somehow get a linear plot in the form of: y = mx + b. The slope would be a constant independent of concentration! 12 We could call the slope the rate constant and assign it the letter k! rate constant = k instantaneous rate or rate “call in the mathematicians” 13 rate constant: k is conc. independent Is still temperature dependent and mechanism dependent! General form of rate law : for RXN: A products rate k[A] m m = RXN order according to A. Determined by experiment only! conc. of A rate constant 14 General form of rate law : aA bB D cC rate k[A] [B] [D] [C] w x y z RXN orders (w, x, y, and z)must be determined by exp. only! Total (overall) order = individual orders 15 General Equation Forms: 0 order: 1st order: 2nd order: 3rd order: rate = k rate = k[A] rate = k[A]2 rate = k[A]3 or rate = k[A][B] or ........ Simple experiments are done by doubling 1 conc. at a time and looking at the effect. 16 General Equation Forms: 0 order: rate = k 1st order: rate = k[A] 2nd order: rate = k[A]2 or rate = k[A][B] 3rd order: rate = k[A]3 or ........ Simple experiments are done by doubling 1 conc. at a time and looking at the effect. order 0 1/2 1 2 doubling [2]0 = 1 [2]1/2 = 1.41.. [2]1 = 2 [2]2 = 4 effect on rate none increase by 1.41.. doubles quadruples Question: suppose rate = k[A]2[B] what is the effect of doubling both A and B? 17 Let’s look a some rate data for the RXN: NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l) 18 NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l) doubles double double doubles 19 NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l) 2 2 2 + [2]x for NH4 : =2 x = 1 (1st Order) 2 for NO2- : [2]y = 2 y = 1 (1st Order) 20 1 4 1 2 rate law : rate k[NH ] [NO ] first order with respect to each reactant, 2nd order overall. orders usually have integer values, but can be fractional. Can also be (-) (inhibitors). Since rate has units, k must also have units. M mol rate time L time so units of k must work with [ ] to match units. 21 Determine the units of k in each of the following: Since rate has units, k must also have units. M rate time (so units of k must work with [ ] to match units.) 1. rate = k[A] M time M M t M2 M M 1 kunits must = time so that : t ? 2. rate = k[A]2 t M M 1 1 kunits must = time M so that : t ? t 3. rate k[NH4 ]1[NO2 ]1 M k MM t so k must have units of M-1t-1 22 4. rate = k[A][B]2[C] kunits=M-3time-1 5. rate = k[A]0 M k units time 23 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) the following data were obtained for the initial rates disappearance of NO: INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Obtain the rate law. What is the value of the rate constant? . 24 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) the following data were obtained for the initial rates disappearance of NO: INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Obtain the rate law. What is the value of the rate constant? Write overall rate equation: rate = k[NO]x[O2]y For NO: select a pair of experiments in which the conc. of [NO] is changed, but other concentrations are unchanged. Let’s use Exp. 1 and 2 25 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO(g) INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Obtain the rate law. What is the value of the rate constant? overall rate equation: rate = k[NO]x[O2]y “Let’s divide Exp.1 by Exp.2 to allow us to cancel terms. x 1 x 2 y 2 1 y 2 2 Exp. 1 rate 1 k[NO] [O ] Exp. 2 rate 2 k[NO] [O ] 26 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO(g) INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Obtain the rate law. What is the value of the rate constant? overall rate equation: rate = k[NO]x[O2]y x 1 x 2 y 2 1 y 2 2 Exp. 1 rate 1 k[NO] [O ] Exp. 2 rate 2 k[NO] [O ] 0.0281 [0.0125]x 0.112 [0.0250]x 27 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO(g) overall rate equation: rate = k[NO]x[O2]y Exp. 1 rate 1 k[NO]1x [O 2 ]1y x y Exp. 2 rate 2 k[NO]2 [O 2 ]2 0.0281 [0.0125]x 0.112 [0.0250]x x 0.0281 0.0125 How do we solve for x? 0.112 0.0250 Use logarithms 0.0281 0.0125 ln x ln 0.112 0.0250 ln0.250 x ln0.5 28 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO(g) overall rate equation: rate = k[NO]x[O2]y x 0.0281 0.0125 How do we solve for x? 0.112 0.0250 Use logarithms 0.0281 0.0125 ln x ln 0.112 0.0250 ln0.250 x ln0.5 ln[0.250] x 2 ln[0.5] Therefore the rate equation becomes: rate = k[NO]2[O2]y 29 Now let’s determine the reaction order with respect to [O2] 30 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) the following data were obtained for the initial rates disappearance of NO: INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Write overall rate equation: rate = k[NO]2[O2]y For O2: select a pair of experiments in which the conc. of [O2] is changed, but all other concentrations are unchanged. Let’s use Exp. 1 and 3 31 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) the following data were obtained for the initial rates disappearance of NO: INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Write overall rate equation: rate = k[NO]2[O2]y Let’s use Exp. 1 and 3 k[NO x3] [O2 ]y3 Exp. 3 rate 3 Exp. 1 rate1 k[NO x] [O ]y 1 21 32 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s Write overall rate equation: rate = k[NO]2[O2]y k[NO x3] [O2 ]y3 Exp. 3 rate 3 Exp. 1 rate1 k[NO x] [O ]y 1 21 0.0561 0.0506 0.0281 0.0253 y y 2 [2] 33 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) Write overall rate equation: rate = k[NO]2[O2]y k[NO x3] [O2 ]y3 Exp. 3 rate 3 Exp. 1 rate1 k[NO x] [O ]y 1 21 0.0561 0.0506 0.0281 0.0253 y y 2 [2] y=1 Therefore: rate = k[NO]2[O2]1 Now solve for k. 34 Ebbing 4th ed. P 490 14.38 In a kinetic study of the reaction: 2NO(g) + O2(g) 2NO2(g) INITIAL CONC. NO Exp. 1 Exp. 2 Exp. 3 0.0125 M 0.0250 M 0.0125 M Overall rate equation: INITIAL Initial rate CONC. of RXN of O2 NO 0.0253 M 0.0253 M 0.0506 M 0.0281 M/s 0.112 M/s 0.0561 M/s rate = k[NO]2[O2]1 Choose any Exp. and substitute in experimental to obtain k. i.e. Exp1. : rate = k[NO]2[O2]1 so : 0.0281 = k[0.0125]2[0.0253] k = 7100 M-2s-1 35 Ebbing 4th ed. P 490 14.40 Iodine ion is oxidized to hypoiodite ion, IO-, by hypochlorite ion, ClO-, in basic solution. I - (aq) ClO- (aq) OH - IO - (aq) Cl- (aq) the following initial rate experiments were run: INITIAL CONC. IExp. 1 Exp. 2 Exp. 3 Exp. 4 0.010 M 0.020 M 0.010 M 0.010 M INITIAL CONC. ClO0.020 M 0.010 M 0.010 M 0.010 M INITIAL Initial rate CONC. of RXN of OHNO (M/s) 0.010 M 0.010 M 0.010 M 0.020 M 12.2 x 10-2 12.2 x 10-2 6.1 x 10-2 3.0 x 10-2 Obtain the rate law. What is the value of the rate constant? . 36 - k[I ][ClO ] rate [OH ] 1 rate k[I ][ClO ][OH ] - k = 6.1 s-1 37 This data isn’t linear! What can we do? Integrated Rate Laws 38 IF we can now somehow get a linear plot in the form of: y = mx + b. The slope would be a constant, independent of concentration! 39 We could call the slope the rate constant and assign it the letter k! rate constant = k rate “call in the mathematicians” 40 Key Equations: Order in [A] 0 1 2 Rate Law* Integrated Rate Law (in y = mx + b form) r at e = k rate = k[A] rate = k[A]2 [A]t = -kt + [A]0 ln[A]t = -kt + ln[A]0 1/[A]t = kt + 1/[A]0 *Since Linear Graph ? vs t [A]t ln[A]t 1/[A]t Slope of Line Equals -k -k k Half life Equations t1/2 = [A]0/2k t1/2 = 0.693/k t1/2 = 1/k[A]0 the units of rate are concentration/time, the units of k (the rate constant) must dimensionally agree. So for each order, k will have different units and those units can tell one which equation to use. [ ] means the concentration of the enclosed species in Molarity (M). 41 The data below was collected for the reaction: NOCl(g) NO(g) + 1/2Cl2(g) Time (s) 0 30 60 100 200 300 400 [NOCl] (M) 0.100 0.064 0.047 0.035 0.021 0.015 0.012 Prepare THREE graphs to determine if the RXN is ZERO, 1st, or 2nd order. Then determine the value and units of the rate constant k. 42 Zero Order Plot [A]t vs. time rate = k [A]t = -kt + [A]0 y = mx + b 43 First Order Plot ln[A]t vs. time rate =k[A] ln[A]t = -kt + ln[A]0 y = mx + b 44 2nd Order Plot 1/[A]t vs. time rate = k[A]2 1/[A]t = kt + 1/[A]0 y = mx + b Plot is linear so 2nd Order k = slope = 0.185 M-1s-1 45 Integrated rate laws: Zero Order: rate = k[A]0 = k rate = k integrated gives: [A]t = -kt + [A]0 y = mx + b slope = -k If a RXN is zero order, a plot of [A] vs. time should be linear and the slope = -k. 46 Integrated rate laws: 1st order rate laws: rate = k[A] integrated gives: [ A ]t ln - kt [ A ]0 rearranged to : y = mx + b gives: ln[A]t = -kt + ln[A]0 slope = -k If reaction data is 1st order, a plot of ln[A] vs. time should be linear. 47 2nd Order Integrated Rate Equations: rate = k[A]2 integrated gives: 1 1 kt [ A ]t [A]0 y = mx + b slope = k If a RXN is 2nd order, a plot of 1/[A] vs. time should be linear and the slope = k. 48 RXN is first order with respect to CH3NC Zero Order Plot 1st Order Plot slope = -k ln[CH3NC]t = -kt + ln[CH3NC]0 49 RXN is 2nd order with respect to [NO2] First order plot 2nd order Plot Slope = k 50 General form 1st order: ln[A]t = -kt + ln[A]0 Note: This is a formula that can be used to solve (1st order) problems. (If all but one of the variables are given) 1. Given the RXN: C3H6 CH2=CHCH3 Where k = 6.0 x 10-4 s-1 @500oC. Looking at the units of k, determine the order. Problem: if [C3H6]0 = 0.0226 M, find [C3H6] @ 955 s. ln[A]t = -kt + ln[A]0 - 6.0 x 10-4 (955s) ln[A]t ln[0.0226] s ln[A]t = -4.362 [A]t = 0.0127 M 51 The other equations can be used in a similar fashion. 52 Half-life: the time it takes to decrease the concentration to 1/2 its initial value. “fold paper to view subsequent half-lives” 53 Half-lives: Formulas: 1st order: t1/2 = 0.693/k Derivation: ln [ A]t kt [ A]0 and: so: ln2 kt1/ @t1/2 : 1 ln kt1/ 2 2 2 ln 2 0.693 t1/ 2 k k 54 Half-life formulas: Zero Order [ A]0 t1/ 2 2k First Order ln 2 0.693 t1/ 2 k k 2nd Order 1 t1/ 2 k[ A]0 These 2 are conc. dependent (and not very useful). 55 Energy Diagrams: All chemical and physical changes are accompanied by energy changes. E = Activation energy energy a reactants E Exothermic products time Question: What keeps the reactants from rolling down the hill? 56 57 58 59 60 61 rate constant (k) varies with temperature. but not with concentration 62 The Arrhenius Equation: Ea /RT k Ae Temp in K 8.31 J/mol•K activation energy base e (natural ln) frequency factor (1/time), fraction of collisions with correct geometry. rate constant -Ea/RT is always <1 and refers to the fraction of molecules having minimum energy for a RXN. 63 Ea /RT k Ae How can we make this a linear equation in the form of y = mx + b? Take the ln of each side. ln k = ln A - Ea/RT or: ln k ln A - Ea 1 R T y = b - mx A plot of ln k vs. 1/T gives a straight line with the slope = -Ea/R (Ea = -8.31 x slope) 64 At two diff. temps. we get: k 2 Ea 1 ln k1 R T1 1 - T2 65 Reaction Mechanisms: Reaction is broken into steps with intermediates being formed. “some RXNS occur in one step, but most occur in in multiple steps.” Each Step is called an elementary step, and the number of molecules involved in each step defines the molecularity of the step. uni-molecular: = 1 i.e. O3* O2 + O bi-molecular: = 2 (these are the most common) i.e. HI + HI activated complex H2 + I2 ter-molecular: = 3 (rare, due to probability of orientation and energy both being correct.) i.e. O(g) + O2(g) + N2(g) O3(g) + “energetic” N2(g)66 The Raschig process for the preparation of hydrazine (N2H4) Overall RXN: 2NH3(g) + NaOCl(aq) N2H4(aq) + NaCl(aq) + H2O(l) Proposed Mechanism: (Only from experiment) Step 1: NH3(aq) + OCl-(aq) NH2Cl(aq) ‡ + OH-‡ (aq) Step 2: NH Cl(aq) ‡ + NH (aq) N H +‡ + Cl-(aq) 2 3 2 5 Step 3: N2H5+(aq) ‡ + OH-‡ (aq) N2H4(aq) + H2O(l) “Cancel intermediates and “add steps” to give overall RXN.” 2NH3(g) + OCl-(aq) N2H4(aq) + Cl-(aq) + H2O(l) The overall rate law, mechanism, and the total order can’t be predicted from the stoichiometry, only by experiment. 67 The following is only true for individual steps: The rate law of an elementary step is given by the product of a rate constant and the conc. of the reactants in the step. Step A Product(s) Molecularity uni rate law rate = k[A] A + B Product(s) bi rate = k[A][B] A + A Product(s) bi rate = k[A]2 ter rate = k[A]2[B] 2A + B Product(s) The overall mechanism must match the observed rate law. Usually one STEP is assumed to be the rate determining step. 68 Example: Overall RXN: 2NO2(g) + F2(g) 2NO2F(g) Observed Experimental rate law: rate = k[NO2][F2] Question: Why does this rule out a single step RXN? Answer: rate law for single step process would be: rate = k[NO2]2[F2] “Let’s try to work out a Mechanism that matches the observed rate law.” 69 Example: Overall RXN: 2NO2(g) + F2(g) 2NO2F(g) Observed Experimental rate law: rate = k[NO2][F2] k1 slow: NO2(g) + F2(g) NO2F(g) + F(g) ‡ rate = k[NO2][F2] k2 fast: NO2(g) + F(g) ‡ NO2F(g) 2NO2(g) + F2(g) 2NO2F(g) The rate law is dependent upon the slow step. 70 The rate law is dependent upon the slow step. Let’s try making the 2nd RXN slow and the first fast Overall RXN: 2NO2(g) + F2(g) 2NO2F(g) Observed Experimental rate law: rate = k[NO2][F2] k1 fast: NO2(g) + F2(g) NO2F(g) + F(g) ‡ k2 rate = k[NO2][F ‡] slow: NO2(g) + F(g) ‡ NO2F(g) 2NO2(g) + F2(g) 2NO2F(g) 71 72 1. At low temperatures, the rate law for the reaction: CO(g) + NO2(g) CO2(g) + NO(g) 2 is:rate = k[NO ] 2 Which mechanism is consistent with the law? a. COrate + NO 2 CO2 + NO rate = k[CO][NO2] b. 2NO2 N2O4‡ N2O4 ‡ + 2CO 2CO2 + 2NO (fast) (slow) c. 2NO2 NO3 ‡ + NO NO3 ‡ + CO NO2 + CO2 (slow) (fast) d. 2NO2 2NO + O2 ‡ 2CO + O2 ‡ 2CO2 (slow) (fast) 73 For the following mechanism: 2NO N2O2 (fast) N2O2 + H2 H2O + N20 (slow) N2O + H2 N2 + H2O (fast) a. Determine the overall reaction b. Does the mechanism agree with the rate law: rate = k[NO]2[H2] 74 75 76 77