Lumbriculus variegatus
advertisement

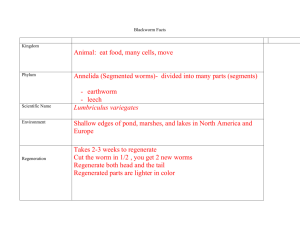
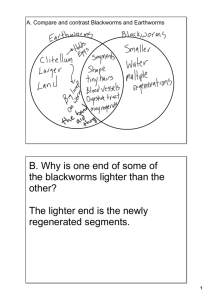
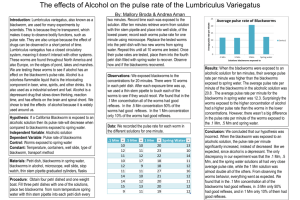
Lumbriculus variegatus Pulse Rate Lab: Stimulant Analysis Introduction: The Lumbriculus variegatus, or blackworm, possesses great scientific value consequent of its unique biological characteristics. Residing in major parts of both North America and Europe, the blackworm can most frequently be observed in marshy areas, or similar places with shallow water, near its favored microhabitats: decomposing foliage, rotting logs, or vegetation near water. The real scholastic significance of the blackworm can be found by observing its closed-circulatory system, made observable by the worm’s transparent skin. Through regular pulsations, the blackworm’s dorsal blood vessel circulates blood and oxygen, via erythrocruorin, the worm’s equivalent to hemoglobin, from the posterior end towards the anterior end. Study of L. variegatus’ pulse rate allows scientists to determine the potential effects a chemical might have on the phylum annelida and humans. Hypothesis: If a blackworm is given stimulant, the pulse rate will be elevated. Independent Variable: exposure to stimulant Materials: 4 Petri dishes, 2 Dependent Variable: pulse rate Plastic pipettes, Microscope, Control: blackworms exposed to spring water Cover Slip, Well Slide, Controlled Variables: ambient temperature, Stopwatch, 10 Blackworms, light level of microscope, feeding frequency, Stimulant Solution Spring relative size of blackworms, worm-handling time Water, Paper Towels Procedure: 1.Collect two groups of five worms in separate containers using two pipettes, one per container. Introduce stimulant to one container and spring water to another. 2.Collect a single stimulant worm into the ‘stimulant’ pipette. Deposit the worm onto a well slide, absorb excess water using paper towel, and cover with a cover slip. Place this setup under a microscope. 3.Turn the microscope’s lamp on to the lowest necessary level. Using the lowpower objective, locate the dorsal blood vessel of the blackworm around the middle third of its body length. 4.Start a stopwatch, and count the frequency of dorsal vessel pulsations for thirty seconds. Double this number, and record in a data table. 5.Using the ‘stimulant’ pipette, collect the worm and place in a new container. 6.Repeat steps 2 through 5 for the other stimulant-exposed worms. Allen Sanford and Michael Patterson 6.Collect a single spring-water worm using the ‘spring-water’ pipette. Deposit on the worm on a new well slide, absorb excess water with a new piece of paper towel, and cover with a new cover slip. 7.Repeat steps 3 and 4 for the spring-water worm. 8.Collect the worm off the well slide, and place the worm in a new spring-water container; use the ‘spring-water’ pipette for handling. 9.Repeat steps 7 through 9 for other spring-water worms. Results: The stimulant group demonstrated a mean pulse rate of 9.2 min-1 higher than the spring water group. Conclusion: Our results display a statistically significant pulse rate increase in the stimulant-exposed worms compared to the spring-water worms. Conventional laboratory practice requires a p-value of less than .05 for a statistical test to be considered “significant”; in a 2-sample t test, our results demonstrated a p-value of .00554, much smaller than necessary. However, this significance is blind to such possible inaccuracies as the doubling of our thirtysecond data and the fact that our control data was collected on a different day than our stimulant data. While not quantitatively affecting the significance, the lab’s results in general are qualitatively weakened by these two impediments. Repeating the experiment with sixty-second trials, more trials per worm, and completion of the procedure unitedly would produce more scientifically viable results. Reference: http://www.eeob.iastate.edu/faculty/DrewesC/htdocs