Unit 2 power point - Boone County Schools
advertisement

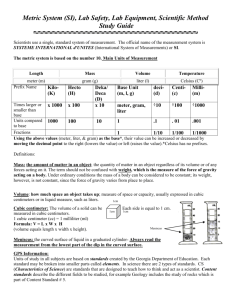
Unit 2:What is Matter? All Matter has 2 Types of Properties of Matter Physical Properties and Chemical Properties 1. Physical properties – can be observed without changing into another substance. State of matter (solid, liquid, gas), texture, color, flexibility, melting and boiling points 2. Chemical properties – describes the ability to change into different substance. They cannot be observed without changing a substance into a new material Able to catch fire (Flammability), ability to react (rusting, tarnishing), nonreactivity (water does not react with air) Measuring Matter Matter is anything that has mass and takes up space (volume) We can measure mass We can measure volume If it can be measured then it is a Physical Property. Measuring Volume Volume- measured in liter or L, mL, and kL or cubic cm (cm³)and cubic m (m³) Volume = L x W x H Measuring Irregular Shaped Objects Displacement – submerge object in water in graduated cylinder. Measure the difference water rises. 1ml of water = 1cm³ Scientist Prefer Mass Instead of Weight Mass – measured in grams or g, mg, and kg Use a balance to measure mass Mass does not change even when force of gravity changes on mass Weight changes with force of gravity; changes with location throughout the universe Use a scale to measure weight Density Density = mass (g) / volume (cm3) If we can measure mass and volume then density is a Physical Property Relative density states that the least dense items will be on top and most dense items on the bottom Entrance Quiz Unit 2 1. Which of the following is NOT a unit of measure for volume? a. cm³ b. ml c. g d. liter 2. What are the 2 properties all matter have? a. mass and weight b. mass and volume c. volume and density d. physical and chemical Entrance Quiz Unit 2 3. This is the amount of matter in an object. a. mass b. density c. matter d. Volume 4. Density is the: a. Amount of space an object occupies b. Force of gravity on an object c. Mass in a given volume d. Amount of mass and space object occupies