Chapter #16 Notes
advertisement

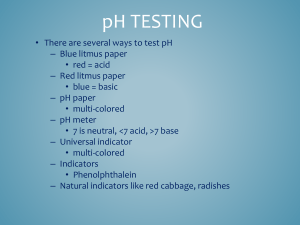
Chapter #16 Acid-Base Titration and pH Chapter 16.1 • In the self-ionization of water, two water molecules produce a hydronium ion and a hydroxide ion by transfer of a proton. • Kw= H3O+ OH• Kw= 1.0 X 10 -14 M2 • The pH of a solution is defined as the negative of the common logarithm of the hydronium ion concentration. • The pH scale 0-14. 7 is neutral…pH<7 acid…pH>7 base • pH= -log ( H3O+) Chapter 16.2 • Acid-base indicators are compounds whose colors are sensitive to pH. • pH range over which an indicator changes color is called its transition interval. • pH meter determines the pH of a solution by measuring the voltage between the two electrodes that are placed in the solution. • Titration is the controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentrations. • Equivalence point is the point at which the two solutions used in a titration are present in chemically equivalent amounts. • End point is the point in a titration at which an indicator changes color. Titration Problems 1. Balance equation 2. Use the known solution to figure out the number of moles (M=mol/L) 3. Use the mole to mole ratio to figure out the moles of the unknown. 4. Determine the Molarity of the unknown using the given volume • “Flask”. May 7, 2007. http://www.chem.ualberta.ca/~iip/chem211i rc/TotalSalt(start).jpg • “Let’s Titrate”. May 7, 2007. http://genchem.rutgers.edu/aanimate.gif • “Titration curve”. May 7, 2007. http://www.cofc.edu/~kinard/Applets/Weak AcidTitration.gif