lecture 10 water in the atmosphere and on earth
advertisement

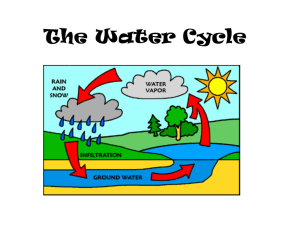
Ice, Water, and Vapor on Earth Water (H2O) is extraordinary in that it coexists on Earth in 3 distinct forms or states, 1: dry solid (ice), 2: wet liquid (water) and, 3: dry, invisible gas (vapor). Nitrogen, N2 and Oxygen, O2 are gases that also exist in liquid and solid forms but on Earth only in laboratories. The photo shows water and ice while the steam plumes suggest water vapor (but are actually condensed water droplets) at Yellowstone Park’s Morning Glory Pool in Winter. Gas – Liquid – Vapor In gases (which are dry and usually invisible), molecules are far apart (so that they have low density) and move freely. In liquids (which feel wet and are visible but often colorless and largely transparent), molecules are clustered together but slide around. In solids (which are visible and may be transparent, but are often colored), molecules are packed together and locked in place, often in crystals. Liquids and solids have much higher density than gases because the molecules are packed closely together. Most solids have slightly higher density than liquids because the molecules are packed more tightly and more efficiently. Temperature and Moving Molecules All gas molecules move rapidly and collide frequently. Collisions ensure that lighter molecules move faster. Also, molecules move faster as T increases. Increasing T increases pressure, but only in a closed container and not in the open atmosphere. Run Program COLLIDE As T rises, atoms and molecules vibrate faster and can become so rapid that different molecules locked together in solids separate, (melting). Increasing T more frees the molecules, which evaporate to gases. Further heating can split individual molecules. The weaker the attractive chemical bonds, the lower the T at which these changes occur. The triangular shape of the H2O molecule gives it extraordinary powers and properties. They shape ice crystals into hexagons that are packed so poorly that freezing expands H2O. The concentration of positively charged H+ ions on one side and negatively charged electrons of the O-2 ion on the opposite side polarizes H2O molecules and makes them act like magnets that can stick (bond) to other H2O molecules. The strong attractions (bonds) of polarized H2O molecules raise the melting and boiling points much higher than for nitrogen, N2 or oxygen, O2 but when they occur deep in the Earth they pull apart the molecules of solid rocks and melt them to magma at much lower T than without H2O. Molecular Structure of Ice = Hexagonal Click to see up-down pattern of each hexagon Top View Side View Adjacent H2O molecules in each hexagon are on different levels or planes. (as if they were in different floors of a split-level house.) Snow and Ice Crystals Each branch is rotated by 60 from the next branch. Spikes on each branch are parallel to the next branch. The Hydrologic Cycle The Hydrologic Cycle is the ceaseless flow of water through the Earth, Oceans, and atmosphere. Fresh water evaporates from the surface of the land and the oceans. The vapor is transported and lifted into clouds in the atmosphere and falls as precipitation. About 2/3 of the water that falls on land evaporates. The rest collects in rivers and flows back to the sea as Runoff, although some water flows underground as ground water. The hydrologic cycle sometimes leads to flood or to drought, but essentially the same water has been flowing through the system’s reservoirs for the past three billion years. Evaporation, the invisible part of the hydrologic cycle, is the power behind the throne of all storms. To see evaporation, fill a wide pan with water on a hot sunny day and watch the water disappear before your eyes. (This happens slowly enough so that you will need to be patient.) The evaporation rate depends on many factors including the 1. Moisture content of the surface 4. Wind Speed 2. Temperature 5. Plant Cover 3. Relative Humidity Precipitation patterns depend on temperature and wind. Since T increases from Pole to Equator, so does precipitation because vapor capacity does. Just as air at the Equator contains about 70 times more vapor than air at the South Pole in January, it also yields 70 times more precipitation. But to get precipitation, air must rise. Air rises where it converges, as in the ITCZ or in low pressure areas, or where the winds are forced to blow upslope against mountains. Air sinks to produce deserts in the subtropical high pressure areas, such as dominate the Sahara. The Hydrologic Cycle: Global Water Fluxes and Supplies Floods and droughts are part of the hydrologic cycle Delaware River Flood 04 April 2005, Milford, NJ http://www.flickr.com/photos/frenchtown-nj/8559476/ Hydrographs are used to predict floods. For a simple quick rainstorm each stream has a standard response. Water rises to a peak some set time after the rain. Double the rain and the flood is twice as large but the time delay is the same. Thus we can time floods for each stream very accurately. Run Storm Hydrograph Current Hydrographs on the Web http://waterwatch.usgs.gov/?m=real&r=us Rural vs Urban Hydrographs Paved areas in cities speed the arrival of rain water to streams. This leads to 1: Quicker rise 2: Higher peak flow 3. Faster recession Thus, urbanization can increase the severity of floods. Rivers and River Basins Egypt’s Nile River Valley and Delta and Sahara Desert (MODIS Image). Amazon River Basin All rain that falls in the light pink area and does not evaporate, flows into the Amazon River. The Amazon has the world’s greatest water volume flow of any river. Its average discharge is 180,000 m3s-1 (cubic meters per second), enough to fill about 2 million bathtubs each second. Determining a River Basin Any River Basin can be determined because it resembles the branches and trunk of a tree. The dashed line or DIVIDE separates different river basins. It is located along the highest elevations (see next Slide). No stream can cross it. http://www.geographyalltheway.com/ib_geography/ib_drainage_basins/the_drainage_basin.htm Havasu Falls, Grand Canyon Near the source of rivers (which may be a spring that comes out of the ground) there are waterfalls and rapids and sediment is eroded. Further downstream the river broadens out onto a floodplain, ow slows and the river begins to meander and ultimately split or braid in a complex of channels, where it deposits the sediments. This often happens when the river nears the sea, where it spreads out into a delta as it empties into the sea. As sediments are deposited in the floodplains or deltas the main river often changes course. The next slides show the act and impact of erosion, deposition, and ground water Alexandre Hogue. Mother Earth Laid Bare, 1936 Dual impact of poor farming practices and a severe Drought - the “Dust Bowl” (ca. 1930-36) on the Great Plains Erosion during downbursts creates gullies in exposed soft sediments of semiarid regions Cappadocia, Turquía Braiding on sediment choked stretch of Alaska’s Matanuska River. Sediments are weathered and eroded by glaciers and running water. The ridge on the right is a moraine or mound of rubble plowed and pushed by an ancient glacier. Braided River, Alaska Groundwater and the Water Table The enormous volume of water stored underground is not stored in vast caverns but in tiny pores between grains of soil or cracks in rock. In sand the fraction of the volume of the pores or Porosity is about 36% of the total volume. Ground water, which we are now depleting, provides the water for hundreds of millions of wells around the world. Mamshit Israel, the Nabatean City of Memphis. The sediment behind the dam has large porosity so it holds much water and also keeps it from evaporating. In that way the Nabateans thrived in the deserts of Israel and Jordan. The Nabateans also built PETRA. Ground Water Nabesna Glacier, Wrangell Mountains, Alaska Note Medial and Lateral Moraines Much of the world’s fresh water is locked up in ice, not all of which is clean Glaciers are like rivers of ice. They form when snow accumulates in the high mountains and gets packed down. The ice then begins to flow slowly downhill, grinding out the valleys and dragging the sediments as lateral and medial moraines. The sediments also pile up into a terminal moraine at the lower end of the glacier, where the ice melts in the so-called zone of ablation. When climate warms the glacier retreats because the melting occurs faster than the downstream flow. Melted glaciers leave behind classical Ushaped valleys (see next slide of Yosemite Valley). Where the ice is brittle it cracks to form large crevasses. This is much like earthquakes. Nearer the bottom the ice is ductile – it flows like plastic. Material buried at the head of the glacier can appear at the foot many years later. This was the case with a plane that was lost over the Andes in 1948 or so. It crashed and has recently reappeared. As ice crystals fall, they pile up on the ground and turn our eyes from the sky above to the ground below, where more beauty and wonder reside. The Antarctic Ice Sheet shows what Yosemite Valley looked like 20000 years ago. Terminal Moraines of Long Island At the height of the last Ice Age about 21000 years ago the ice sheet covering Canada and the northern USA reached Long Island (which was then not an island) and left behind a long rubble hill or moraine, now up to 400 feet above sea level. Long Island, as a result is a long ridge of loose dirt and rocks. http://people.hofstra.edu/J_B_Bennington/research/long_island/li.html Block Island Lighthouse – Cliff is the Remnants of the Terminal Moraine Water vapor is the most variable gas in the atmosphere because it readily evaporates from water or sublimes from ice, and then condenses to water or deposits to ice. And since water is the staff of life, vapor is often treated differently from all other gases in the atmosphere even though it is just a gas. The way to condense vapor is to cool the air. This happens every night near the ground, on the grass (where dew is formed). Beautiful strings of dew drops that condense at night often appear on spider webs. (see next slide.) One way to remove almost all vapor from the air is to chill it by pumping air through a pipe immersed in dry ice or liquid nitrogen (see 2nd slide). The vapor deposits or crystallizes directly to ice inside the pipe. Droplets condensing on a spider web overnight Measures of Vapor and Definitions of Humidity Temperature (T)is a measure of the kinetic energy of the molecules. Two objects have the same temperature when no heat is transferred between them when they are brought into contact. Mixing Ratio (w) is the mass fraction of vapor in the air. It is often expressed as the number of grams of water vapor per kilogram of dried air, or parts per thousand (‰). Vapor Pressure (e) is the part of air pressure due to the pressure of the water vapor molecules. When divided by air pressure, it gives the fraction of vapor molecules in the air. Relative Humidity (RH) is the vapor content of the air divided by the vapor capacity expressed as a percent (%). Saturation occurs when the vapor content of the air is at its capacity. Any more vapor quickly condenses into water or deposit to ice. When air is saturated with water vapor RH = 100% Dew Point Temperature (Td)is the temperature at which the air becomes saturated after cooling without adding water vapor. Further cooling causes cloud droplets or dew (condensation). Wet Bulb Temperature (Tw) is the temperature at which the air becomes saturated after cooling by evaporating water. (When RH < 100%, Td < Tw < T). Relative Humidity Relative humidity (RH) increases when vapor content increases or vapor capacity decreases. Since vapor capacity increases with T, falling T mean falling vapor capacity and rising RH. Ah! Now I finally understand why RH is lowest in mid afternoon and highest around dawn, when fog and dew are most likely. High T Large Capacity Low T Small Capacity Vapor Content Constant Morning Low T, High RH Afternoon High T, Low RH Condensing Vapor – Producing Precipitation Vapor Capacity All clouds and rain are formed by the process of cooling air. As air cools its water vapor capacity decreases. When vapor capacity falls below the original vapor content the excess vapor condenses to form liquid water or ice. 20 12 13 12 9 9 7 7 6 6 Vapor Content HOT 6 3 5 Cooling….Produces Condensed Water COLD Vapor Pressure and Condensation 2500.0 Vapor Pressure (Pa) Vapor capacity roughly doubles for every T increase of 10ºC. 2000.0 es(T) 1500.0 Excess Vapor Condensed Original Td 1000.0 Original Vapor Content 500.0 When air is cooled below the saturation point, (i. e, dew point, Td) the excess vapor condenses 0.0 273 278 283 288 293 Temperature (K) The next Slide shows how to calculate the Amount of Water Vapor that condenses when air is cooled. Vapor Fraction (w), Relative Humidity (RH), and Condensation of Vapor. The table shows the saturated (capacity) mass percent of water vapor in air at p = 105 Pa ( sea level pressure). Thus,, 100 g of air at 20C can hold 1.49% × 100 g = 1.49 g of vapor. [300 g of air at 20C can hold 1.49% × 300 g = 4.47 g of vapor]. RH equals actual vapor content (wact) divided by vapor capacity (wsat). Example 1: Find RH of 100 grams of air at T = 20C that actually contains wact = 0.5 grams of water vapor. Solution: RH w content 0.5 act 33.6% capacity wsat 1.49 Meteorologists must predict how much rain or snow will fall. To do this they must calculate how much vapor will condense when air is cooled. If final vapor capacity, wsat(final) is less than initial vapor content, wact(initial) the amount that condenses is the difference between the two times the mass of air. Example 2: If the air in Example 1 air is chilled to T = -10C calculate how much vapor will condense. Solution: w actinitial wsatfinal mair 0.5% 0.179% 100 g 0.321 g T(o C) % wsatl(liq) *% wsatl(ice) 40 4.957 0 35 3.708 0 30 2.756 0 25 2.034 0 20 1.488 0 15 1.078 0 10 0.772 0.00 5 0.546 0.00 0 0.382 0.382 -5 0.263 0.251 -10 0.179 0.162 -15 0.119 0.103 -20 0.078 0.064 -25 0.050 0.040 -30 0.032 0.024 -35 0.020 0.014 -40 0.012 0.008 -45 0.007 0.005 -50 0.004 0.002 * At T < 0 C there are two values for vapor capacity – a lower value (wsat(ice)) if ice is present and a higher value (wsat(liq)) for supercooled liquid water. When both supercooled droplets and crystals are present , crystals will steal vapor from droplets and grow at their expense! Supercooled Water, Ice, and Precipitation The charts and tables on the previous slides show that when T < 0C, vapor capacity at the surface of ice is less than at the surface of liquid water because fewer vapor molecules have the additional energy they need to escape from ice. This difference implies that when supercooled water (water that remains liquid below 0C) and ice are both present in a cloud, ice crystals will grow at the expense of the supercooled droplets (which will shrink and evaporate). The ice crystals then grow large enough to fall, as we see in cirrus clouds or in the altocumulus clouds on the next slide. This mechanism, called the Wegener-Bergeron process is responsible for most rain except in air over the tropical oceans. Alfred Wegener (of Continental Drift fame) was the first to point out that ice reduced vapor capacity and Tor Bergeron realized it accounted for most rain produced outside the tropics In the case of the altocumulus clouds shown in the next slide, the supercooled water droplets freeze when an airplane flies through the cloud and chills the air around the wingtips. Ice crystals grow at the expense of supercooled droplets and fall out of the cloud.