Chapter 18 * The Gas Laws
advertisement

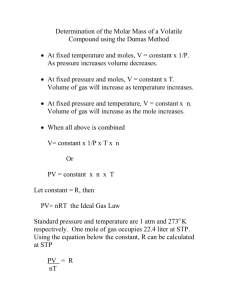
Chapter 18 – The Gas Laws Section A – Gas Laws Boyles Law Charles Law Gay – Lussac’s Law Avagadro’s Law Section B – The combined gas Law P1 = P2 = V1 = V2 = T1 = T2 = *** Convert Celsius to Kelvin by St.Dominic’s College Chemistry notes Page 1 Chapter 18 – The Gas Laws Example 1 If a definite mass of gas occupies 500 cm3 at a pressure of 101,000 Pa and a temperature of 27 oC, what is its volume in cm3 at s.t.p.? (Standard temperature = 0 oC, standard pressure = 101,325 Pa) Section C : The Kinetic theory of Gases Assumptions Gases are made up of particles whose ___________ are ______________ compared to the _____________ between them. There are no __________ or _____________ forces between particles. Particles move in a ___________ motion, ____________ with each other and container walls. The average ____________ energy of the particles is _____________ to the Kelvin temperature. All collisions are perfectly _____________ ( speed of a particle before and after a collision are the same) Ideal gas St.Dominic’s College Chemistry notes Page 2 Chapter 18 – The Gas Laws Real gases deviate from ideal behaviour Real gases deviate most from ideal behaviour when they are in conditions of __________ temperature and __________ pressures Particles now have less kinetic energy ( moving more slowly) and are pushed closer together and do ____________ and ___________each other The particles do have an ____________ _____________. **** Gases which behave most like ideal gases will be the ones with the ____________ intermolecular forces between particles. Think which of the following gases would be most like an ideal gas? – H2, HF, NH3 and F2 Section D: The equation of state for an ideal gas P V n R= 8.31JK-1mol-1 T Example 1 How many moles of nitrogen gas are present in 200cm3 of the gas at 35oC and a pressure of 200kPa? (The gas constant is 8.31JK-1 mol-1 ) St.Dominic’s College Chemistry notes Page 3 Chapter 18 – The Gas Laws Section E: Mandatory experiment: Determination of the relative molecular mass of a volatile liquid using the ideal gas equation PV= nRT A volatile liquid is a liquid with a ___________-__________point. Example: A volatile liquid must be used in this experiment because it has a boiling point of less than __________ which is the boiling point of water. Procedure 1) Find the total ____________ of a dry clean conical flask, a circle of aluminium foil and a rubber band. 2) Add about 3cm3 of a _____________ liquid into the flask. 3) Cover the flask with the aluminium foil using the rubber band and make a small hole in the foil using a _____________. 4) _________________ the flask into a beaker of ___________water. 5) The liquid inside the flask will all _____________ and some of it will escape through the hole in the foil. This will continue to happen until_________________________________________________________________ ____________________________________________________________________. 6) Remove the flask from the water when____________________________________. 7) Record the temperature of the hot water using a ___________________________ **( this is the temperature of the _______________________) 8) Record the atmospheric pressure in the room using a __________________ ***( this is the pressure of the _____________________) 9) Allow the flask to cool. You will notice_____________________________________ ____________________________________________________________________ 10) Dry the outside of the flask, and then find the mass of the flask, rubber band and the foil. ****( The mass of the vapour is now found by ___________________________________________________________________) 11) Remove the foil and band. Find the volume of the flask by filling it with water and then measuring the volume of the water with a ________________ cylinder. ***** ( This is the volume of the __________________) 12) Calculate the value of the relative molecular mass of the gas using the equation PV= nRT St.Dominic’s College Chemistry notes Page 4 Chapter 18 – The Gas Laws Example 1 In order to determine the relative molecular mass of a volatile liquid a conical flask was filled with vapour of the liquid. The following results were obtained: Atmospheric Pressure = 100000Pa , Volume of flask = 0.000315m3, Mass of vapour – 1.1g , temperature of the vapour = 300K Calculate the relative molecular mass. ( To the nearest whole number) (The gas constant is 8.31JK-1 mol-1 ) Answer: Part 1 – List all quantities needed for the equation in the correct units P = Pressure = 100000Pa , V = Volume = 0.000315m3, n = number of moles = ?, R = gas constant = 8.31JK-1 mol-1 T = temperature = 300K Part 2 – Sub into the equation PV = nRT (100000)(0.000315 ) = n (8.31) (300) (100000)(0 .000315) = n (8.31) (300) 0.0126 = n ( the number of moles) Part 3 – Find the RMM X Relative Molecular Mass Mass g Moles ÷ Relative Molecular Mass 0.0126 moles x RMM = 1.1 g RMM = 1.1/ 0.0126 RMM = 87.3016 To the nearest whole number – RMM = 87 St.Dominic’s College Chemistry notes Page 5 Chapter 18 – The Gas Laws Check your learning of Gas Laws Green = I know this already Orange = I am not sure – have to revise this Red = I don’t know this yet – have to learn it Can you Green Orange Red State Boyle’s law. State Charles’s law. State Gay-Lussac’s law of combining volumes (higher level only) State Avogadro’s law.( higher level only) Know that the combined gas law is used to calculate changes in temperature , pressure or volume of a gas and know how to use it All of the below are Higher level only : Be able to explain what the kinetic theory of gases is about Know what an ideal gas is Know how real gases deviate from ideal behaviour Equation of state for an ideal gas: PV = nRT (units: Pa, m3, K). Know how to do calculations to find any missing part Find the Relative Molecular mass of any gas ( once the mass is given) Using PV = nRT (units: Pa, m3, K). Mandatory experiment – to calculate the relative molecular mass of a volatile liquid using the ideal gas equation. St.Dominic’s College Chemistry notes Page 6