Specific Heat power point presentation
advertisement

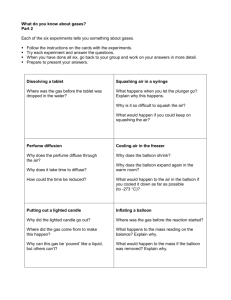
Bell Ringer Balance the following combustion reaction. Also, name the hydrocarbon and draw the hydrocarbon structure. Do Now You heat a balloon filled with air. What do you think happens to the pressure inside of the balloon? Why? By the end of the day today, SWBAT… • Explain and calculate specific heat Why it matters in LIFE: Explains why certain objects are made out of certain materials Why it matters in THIS CLASS: It connects everything we have done thus far in quarter 3– you will have a mastery quiz on __Specific Heat__ so let’s hit Specific Heat Specific heat is a property of matter that describes the amount of energy required to raise the temperature of one gram of a substance by 1 °C Specific Heat Specific heat is a constant that relates heat and temperature change per kilogram Different materials have a different specific heat A low specific heat means heat is conducted through an object quickly Specific Heat CONDUCTORS: change heat easily Low Specific Heat Require little energy to change temp INSULATORS: Do NOT change heat easily High Specific Heat Require LOTS of energy to change temp Example! Aluminum vs. Stainless Steel Low specific heat capacity = substance needs less heat substance needs to get hot High specific heat capacity = substance needs more heat a substance needs to get hot Aluminum is 0.9 J/kg C Stainless steel is 0.5 J/kg C Material C (J/kg-C) Water 4184 Plastic Foam 2010 Air, 200 K 1650 Aluminum 897 Iron 449 Brass 375 Conductors Insulators Specific Heat Specific Heat Demo I have two balloons – one filled with water and one filled with air What should happen to the balloon? Specific Heat Demo Why did the balloon filled with water not explode? The water balloon does not explode because the water inside absorbs the heat better than the air does Water has a HIGHER specific heat, which means it has a HIGH heat capacity It takes a lot more energy to change the temperature of the water than it does for the air Specific Heat – Equation Q = mCDT Q = mC(Tf – Ti) Q = heat (joules-J) m = mass (kg) C = specific heat (J/kg C) DT = change in temp (C) Example 1 Q = mCDT If the specific heat of methanol is 251 J/kg-˚C, how many Joules of heat are needed to raise the temp of 0.250 kg of methanol from 18˚C to 33˚C? Example 2 Q = mCDT How much does a piece of ice weigh if it absorbs 200 J of heat when it is melted from 0˚C to 25˚C? Group Practice — Area 1 A .625 kg sample of water was cooled from 50˚C to 10˚C. How much heat was lost? The specific heat of water is 4,184 J/kg-˚C. Group Practice — Area 2 The heat capacity of lead is 130 J/kg-˚C. How much heat (in J) is required to raise the temperature of 0.015 kg of lead from 22˚C to 37˚C? Group Practice — Area 3 What is the specific heat of a substance that absorbs 250 J of heat when a sample of 10 kg of the substance increases in temperature from 10˚C to 70˚C? Classwork Work a s group to complete the class work activity. This is the time to ask questions and try to get a better understanding of this topic. Exit Slip Expectations Complete the exit ticket questions INDEPENDENTLY You are not getting enough INDIVIDUAL practice before assessments, which is one of the reasons why the grades are so low You don’t really know it unless you can do it ON YOUR OWN Exit Slip The specific heat of SrCl2 is 0.5 J/gC. How much heat is needed to change the temperature of a 20 gram sample of SrCl2 from 10˚C to 20˚C? Specific Heat Quiz 1. When 3.0 kg of water is warmed from 10.0°C to 80.0°C, how much heat energy is needed? 2. 1960 J of heat are added to 500. g of copper taking its temperature from 25.0 ºC to 35.1ºC. What is the specific heat capacity of copper? 3. Calculate the temperature change when 10,000.0 g of water loses 232,000 J of heat.