Primer - Workforce Development in Stem Cell Research
advertisement

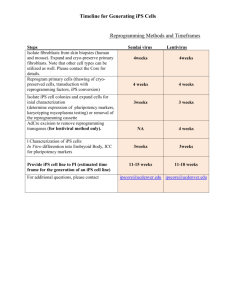
Primer for Reprogramming Lecture Essential questions (R, B and V) What das Nuclear Reprogramming mean (definition)? How can you reprogram a somatic cell towards pluripotency? Does reprogramming occur in nature? If so, where? What are the main differences between the reprogramming strategies? Is an ES cell identical to an iPS cell? What are the Yamanaka factors? What happens with a somatic cell during reprogramming with regard of its genome? Key knowledge and skills will you acquire as a result of this lecture Students will know: Key Terms: embryonic, and induced pluripotent stem cells, somatic nuclear transfer, cell fusion, direct reprogramming, transcription factor, ectopic expression, epigenome, epigenetic modification The history of nuclear reprogramming The Yamanaka factors and the basic function of OCT3/4 and SOX2 The basic difference between iPS cells and ES cells The different nuclear reprogramming towards pluripotency strategies and their differences The therapeutic potential of RiPSCs The basic molecular events during nuclear reprogramming Students will be able to: Describe the potential and advantage of iPS cells in contrast to ES cells for stem cell research (drug development, biology of pluripotency, disease modeling) Describe the basic events that happen after forced expression of transcription factors Highlight the different methods of reprogramming and the key factors that lead to the transition for each strategy. Recognize the potential to study stem cell biology (factors necessary for pluripotency, self-renewal, etc.) for each reprogramming method As cells proceed in normal development from embryonic and fetal stages to an adult state, they become increasingly committed to their differentiated state. Hardly ever do cells reverse this process and go back from a differentiated state to an embryonic one. The process of mammalian cell differentiation can be described as a ball rolling down a hill with many valleys [The landscape model of mammalian cell differentiation (modified from Keeton & Gould 1984)]. When the ball is on tip of the hill, it can roll down through any valleys below; this represents the process of a totipotent cell that can differentiate into any tissue of the body. However, as the ball rolls passed an intersection, the available valleys for the ball to roll down become limited. When the ball reaches the bottom of the hill, it can no longer move to another valley or back to the tip of the hill. This model was used to illustrate a totipotent cell choosing among different developmental paths; when the cell’s fate is partially determined, its differentiation potential becomes limited (slide 2). In the current literature, the term “nuclear reprogramming” is defined as either the switch of the gene expression state from one cell type to another or the change of a differentiated, specialized cell into a developmentally more primitive but more pluripotent state (slide 2). Until the early 1950s the validity of the dogma, that the process of a cell, to differentiate and eventually commit to its fate, is irreversible has not been questioned. However, early studies in frog cloning starting in 1952 demonstrated that the process of cell differentiation could be fully reversed without the alteration of the gene content (slide 4). Soon after this first experimental evidence for reprogramming, a number of different techniques have been developed over the years to induce reprogramming and pluripotency in differentiated somatic cells including nuclear transfer, cell fusion, reprogramming through cell extracts and direct reprogramming (slide 3). Latter has drawn much attention and undergone dramatic advancement and improvements since first described in 2006. To produce pluripotent cells using nuclear transfer, the nucleus of a somatic cell (e.g. fibroblast) is removed (slide 6). At the same time, the nucleus of an egg cell (oocyte) is taken out and discarded. The nucleus of the somatic cell is then inserted into the enucleated egg cell. After insertion into the egg, the somatic cell nucleus is reprogrammed (i.e. has its gene expression altered, turning OFF genes marking its differentiated state and turning ON genes that induce pluripotency) by unknown factors present within the oocyte. The egg, now containing the nuclear DNA of the somatic cell, is stimulated with electricity and begins dividing. After several mitotic divisions in culture, a blastocyst may form from which pluripotent stem cells can be derived. This process is very inefficient in species where it does work, and as of this writing has never been accomplished using human cells. The technique of transfering a nucleus from a somatic cell into an oocyte is also referred to as cloning. Importantly, these NTES cells contain the same genetic material (DNA) as the original fibroblast cells (i.e. those taken from the patient’s skin) and may in theory be used for therapeutic purposes in a manner that would avoid immune system rejection. As described above, such cells made from patients may be differentiated into specific lineages (e.g. dopaminergic neurons) to enable the study of a particular disease (e.g. Parkinson’s disease). Currently no human ES stem cell lines have been derived using this method. This technique is not very widely studied due to: 1) the considerable difficulty, both practically and ethically, in obtaining human eggs, and 2) the advent of iPS technology (see below). Cell fusion describes a process of forcing several nuclei to share a common cytoplasm, leading to multinucleated cells or hybrid cells (slide 8). In multinucleated cells, so called heterokaryons, one cell type usually dominates over the other, such that it imposes its state on another nucleus, where eventually nuclear reprogramming occurs. Fusion of somatic cells with pluripotent cells and initiating reprogramming is a direct and fast method (1-2 days). Another method to generate pluripotent stem cells is through a technique called direct or cellular reprogramming (slide 10). Induced pluripotent stem cells (iPS cells) are reprogrammed cells made by a technique that “forces” expression of pluripotency-related genes in a somatic differentiated cell (slide 18). iPS cells are similar to ES cells in many respects, such as their pluripotency and matching expression of genes and proteins (slide 18). iPS cells show genome-wide histone methylation patterns characteristic of ES cells, identical morphology, expression of key pluripotency markers and even reactivation of the inactive X chromosome and restoration of telomerase activity. Despite the functional and molecular similarity between ES and iPS cells (with the tetraploid (4N) complementation assay being the most stringent), iPS cells are not identical to ES cells in terms of global gene expression, epigenetic modifications and germ line transmissibility. iPS cells were first produced in 2006 from mouse cells with the forced expression of the Yamanaka factors and in 2007 from human cells (slide 10). This was an incredibly important and monumental advance in biology, as it not only opened the door to a much clearer understanding of how pluripotency is biologically regulated but may also allow researchers to obtain pluripotent stem cells which are a genetic match for patients, without the controversial use of human embryos. iPS cells are typically derived by introducing specific pluripotent stem cellassociated genes into non-pluripotent cells such as adult fibroblasts, by way of a viral vector in a process called transduction (slides 12). This is often achieved using a type of virus called a retrovirus. Retroviruses have an RNA genome that is converted to DNA before inserting at random locations into the host cell’s own genome. The reprogramming genes include the master transcription factors Oc3/4 and Sox2. The Oct3/4 and Sox2 transcriptional regulators and are necessary to induce somatic cells into an embryonic state. Oct-4 plays a crucial role in maintaining ES cell pluripotency, and is primarily found only in pluripotent cells such as ES cells (slide 20). The absence of Oct-4 in blastomeres and ES cells leads them to change into trophoblast cells. The Sox2 gene is also associated with maintaining pluripotency (slide 21). Klf4 was initially required for mouse iPS cell production but was later shown not to be required for human iPS cells. The c-Myc proto-oncogene has been implicated in cancer. Using the "myc" family of genes to induce iPS cells is troublesome because 25% of mice transplanted with c-myc-induced iPS cells developed lethal tumors. Klf-4 and cMyc work by changing gene expression in differentiated cells, primarily by pushing cells to proliferate, preventing cell death and making the genome more responsive to changes in patterns of gene expression. Reprogramming factors turn OFF genes that are active in differentiated cells and turn ON genes that maintain pluripotency. Soon after, the work by the group has been developed and optimized from various point of views, including the demonstration that the usage of a different set of reprogramming factors, namely the "Thomson factors" (OCT3/4, SOX2, NANOG and LIN28) also leads to the derivation of iPS cells in humans. In ES cells, Nanog is also required for pluripotency. However, Nanog is not necessary for iPS induction, although human iPS cells are often produced using Nanog as one of the factors. Lin-28 is a regulator of a specific class of factors known as microRNAs, that in turn regulate many oncogenes. Several other genes such as hTERT and SV40 or the combination of the Thomson and Yamanaka factors increase the production efficiency of iPS cells. Though cellular reprogramming superficially appears “simple” considering that very few genes are required to push mature cells “developmentally backwards” into pluripotency, in reality the process is anything but simple when one considers the many interactive regulatory networks affected by each of these factors during the reprogramming process. Reprogramming usually takes between 2-4 weeks after reprogramming genes are introduced to cells. Small numbers of cells become morphologically and biochemically similar to pluripotent stem cells, and are isolated through morphological selection (formation of colonies), the presence of a genetic marker or antibiotic selection (slide 16). A variety of innovative techniques have been shown to reprogram somatic cells into pluripotent stem cells since 2006 including the use of viruses, proteins, plasmids and mRNA molecules (slide 11, 17). In addition, different cell types have been reprogrammed including skin fibroblasts, keratinocytes, and blood cells (slide 22). Reprogramming adult cells to obtain iPS cells may pose significant risks that could also limit their use in humans. If viruses are used to alter the cells genetically, the expression of cancer-causing genes or oncogenes may be activated after they are injected into organisms. In February 2008, scientists developed a technique to remove these reprogramming genes after inducing pluripotency, increasing the potential safety of iPS cells for the treatment and study of human diseases. In April 2009, scientists produced mouse iPS cells without any genetic alteration of adult cells. Here, a repeated treatment of the cells with critical proteins was enough to induce pluripotency (slide 13). The most recent development within this field comes from a research group at Harvard University. The Rossi laboratory accomplished to reprogram fibroblasts from different sources into iPS cells by transfecting them with synthetic mRNAs, each encoding the different reprogramming factors (slide 15). To date, this is the only study that demonstrates that this new methodology is a robust and efficient way of generating iPSCs without compromising genomic integrity. By such means, today’s breakthroughs are refined further to create even more effective methods for eventual use in cellular therapies. Little by little, the processes behind cellular reprogramming of somatic differentiated cells into induced pluripotent stem cells are being discovered and more detailed studies of the molecular mechanisms behind are being undertaken. Nuclear reprogramming is an incredibly complex and dynamic process likely to follow a specific set of sequential events involving epigenetic changes combined with the conversion of an entire transcriptional network (slide 24). An epigenome of a cell consists of a record of the chemical changes to the DNA and histone proteins of an organism; these changes can be passed down to an organism’s offspring. Changes to the epigenome can result in changes to the structure of chromatin and changes to the function of the genome. Epigenetic changes of the genome are controlled by DNA methylation, histone modifications, and in a broader sense, small, noncoding RNAs. Both DNA methylation and histone modifications affect the structure of the chromatin rather than the sequence. Thus, these modifications can be erased and reestablished without mutating the genome. DNA is methylated on the cytosines of CpG dinucleotides by DNA methyltransferases (DNMTs). The extent of cytosine methylation, predominantly on CpG islands of promoters, reflects the expression status of the gene, with hypermethylation keeping the gene silent and hypomethylation allowing high gene expression (slide 24). In differentiated cells, many developmental and differentiation associated genes are active and show enrichment for H3K4me3 and lack of DNA methylation. Some early developmental genes have been silenced through polycombmediated H3K27me3 and all pluripotency associated genes show high levels of DNA methylation. In pluripotent cells, these pluripotency-associated genes are active and show H3K4me3 and lack of DNA methylation. Many of the developmental genes show a ‘bivalent’ chromatin configuration and tissuespecific genes tend to be DNA methylated. It is believed that the process of nuclear reprogramming may involve a sequence of stochastic epigenetic events and involves sequential activation of pluripotency markers (slide 25). In mice, alkaline phosphatase (AP) and SSEA1 positive cells are already detected 3 and 9 days, respectively, after viral reprogramming factor transduction, whereas GFP expressed from the endogenous Oct4 or Nanog loci first appear only after 2 weeks. The virally transduced factors need to be expressed for about 2 weeks to initiate the reprogramming process. Another model suggests that reprogramming may be described as a two-stage process (slide 25). In the first stage, exogenous Oct4 and Sox2 cause the downregulation of lineage-associated genes and upregulation of a subset of ES cell-specific genes. In addition, the first stage of reprogramming comprises widespread epigenetic remodelling: epigenetic enzymes that are most likely activated by reprogramming factors induce a global unfolding of chromatin and catalyze the removal of repressive chromatin modifications from key pluripotency genes. This allows these pluripotency genes to be targeted and activated by exogenous Oct4 and Sox2, resulting in the revival of the interconnected autoregulatory loop and reactivation of the ES cell transcriptional network. At the same time, transgene silencing that was initiated during the first reprogramming stage reaches completion, leading the fully re-established pluripotent state to be independent from continuous transgene expression In all proposed models exogenous Oct4 and Sox2 resuscitate the interconnected autoregulatory loop during reprogramming (slide 26). In infected fibroblasts, endogenous Oct4, Sox2 and Nanog are reactivated by ectopic expression of Oct4, Sox2 and other reprogramming factors. Progression of the reprogramming process results in increased selfsustainability of the endogenous interconnected loop. The interconnected loop is now resuscitated and is able to stably maintain the pluripotent state. Concept Mapping Terms • • • • • • • • • • • Induced pluripotent stem cell Nuclear transfer Transcription factor Direct reprogramming Reprogramming factor Epigenome Transgene Transcriptional Regulatory Circuitry Yamanaka factors Oncogene Methylation Readings, Videos, and Slide Presentations for Lecture Nuclear Reprogramming Please watch a series of movies from the International Society for Stem Cells (http://www.isscr.org/public/MakingSenseOfStemCells.htm) regarding human embryonic stem cells, adult stem cells and their therapeutic use and cloning and nuclear transfer. References Nuclear Reprogramming and Stem Cells (Stem Cell Biology and Regenerative Medicine) by Justin Ainscough, Shinya Yamanaka and Takashi Tada (Hardcover - Sep 1, 2011) Stem Cells: Nuclear Reprogramming and Therapeutic Applications, Novartis Foundation Symposium (Novartis Foundation Symposia) by Novartis Foundation (Hardcover - May 16, 2005) Cellular Programming and Reprogramming: Methods and Protocols (Methods in Molecular Biology) by Sheng Ding (Hardcover- Mar 25, 2010) Engineering of Stem Cells (Advances in Biochemical Engineering Biotechnology) by Ulrich Martin (Hardcover - Oct 27, 2009) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming by R. Jaenisch and R. Young (2008) The molecular mechanism of induced pluripotency: a two-stage switch by W. Scheper and S. Copray (2009) Induced pluripotent stem cells: current progress and potential for regenerative medicine by G. Amabile and A. Meissner (2009) Nuclear reprogramming to a pluripotent state by three approaches by S. Yamanaka and H.M. Blau Totipotency, pluripotency and nuclear reprogramming by S. Mitalipov and D. Wolf Biological Science by Keeton W & Gould J (1984, New York: WW Norton and Company, Inc.)