Chapter11-Nuclear En..
advertisement

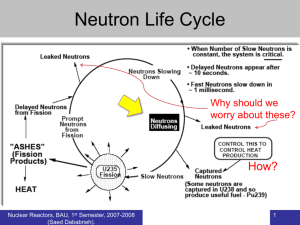
Nuclear Energy Fossil Fuel vs. Nuclear Fuel Fossil Fuel: Provides energy by chemical reactions (No change in atoms) Uranium: There is a change in structure of atom (energy release I given in terms of binding energies) ENGR302I Atom Atomic Number = # of protons (P) Mass Number =# neutrons + protons (A=n+P) The nucleus of an atom of carbon has 6 protons and 8 neutrons ENGR302I Binding Energy per Nucleon Fission (breaking up the heavy atoms) Fusion (fusing the light atoms) ENGR302I Isotopes Isotopes are unstable atoms having the same number of protons, but different number of neutrons (different A and same P). U-235 has 92 protons and 143 neutrons, U-236 has 92 protons and 144 neutrons. Normal hydrogen has 1 proton and 0 neutron, deuterium has 1 proton and 1 neutron, tritium has 1 proton and 2 neutrons Nomenclature ( number of protons) 92 ENGR302I U235 (atomic mass number) Isotopes Isotopes are chemically identical but physically are very different Isotopes are radioactive Isotopes are rare 234 92 U 235 92 U 238 92 U 1 1H 2 1H 3 1H (0.006%) (0.714%) (99.28%) ENGR302I (99.80%) (0.15%) (0.05%) Fission History 1896 – Antone Bequerel (France) uranium salt darkened photographic plates even in total dark 1900 – Maria Curie (Poland) 1911 - Ernest Rutherford (New Zealand) radioactivity consisted of three components first planetary model of atom of hydrogen 1945 - First atomic explosion Alamogordo, New Mexico, July 16 1945 – Fist Atomic Bomb Hiroshima, August 6 ENGR302I U.S. Nuclear Industry 103 Power plants as of 1996 providing 20 % of the US electric power 90% are in Northeast and Midwest Oil Embargo – 1973 No new plants since - 1976 Accident at Three Miles Island - 1979 Operating licenses for many power plants will expire in the next ten years. ENGR302I Nuclear Plants ENGR302I Fission ENGR302I Basic Physics Reactions U-235 + n U-236 Many ways that U-236 can decompose, e.g. U-236 Ba-137 + Kr-97 + 2n + energy U-236 Xe-140 + Sr-94 + 2n + energy Energy is in the form of gamma-rays ENGR302I Nuclear Energy Energy E= Dm. C2 Nuclear energy from 1-kg of uranium = Chemical energy from 2000 tons (2 million kg) of coal Mass is usually expressed as atomic mass unit 1 amu= 1/12 of the mass of C-12 atom=1.66x10-7 kg ENGR302I Uranium Uranium ore is only .7% U-235, rest is U-238 U-235 must be enriched to 3% before it can be used as nuclear fuel. Only U-235 is fissionable (fertile). If neutron not acquired by U-235, it will be acquired by U-238 The slower the neutron, the more chance that U235 will acquire it. ENGR302I Fuel Cycle ENGR302I Nuclear Power Plant ENGR302I Basic Components of Conventional Nuclear Reactors Fuel Rods (3% U-235, 97% U-238) Control Rods (Boron/ Cadmium) Moderator (Graphite / Water) B-10 + n Li-7 + He-4 If neutron is too fast short contact time If neutron is too slow not enough energy Coolant (Water / Sodium) ENGR302I Types of Reactors Coolant Moderators Water, Graphite Pressure LW, HW, Gas, Liquid Metals Low Pressure (LWR) Pressurized (BWR) Fuel Uranium-235 Plutonium-239 MOX ENGR302I Classification Light Water Reactors Pressurized Water Reactors Boiling Water Reactors High Temperature Gas Cooled Reactors Fast Breeder Reactors Pebble Bed Modular Reactors ENGR302I Breeder Reactors Convert Non-fissionable U-238 to fissionable Pu-239 Can use either U-235 or Pu-239 U-238 + n Np-239 Pu-239 Mainly in Europe and Russia Must use sodium (instead of water) as coolant/moderator Does not slow down neutron (as water does) Much higher heat capacity Disadvantages are: Sodium is highly explosive Plutonium is bomb-grade quality ENGR302I Breeder Reactors 238U + n 239U 239Np 239Pu uranium-238 + neutron plutonium-239 ENGR302I Pebble Bed Reactors ENGR302I Nuclear Safety Nuclear Regulatory Commission is in charge of all nuclear safety issues Major factors are: Design Steel-reinforced containment must withstand severe hurricanes and earthquakes, and direct hit by a large jetliner Multiple backup systems must be in place Automatic shutdown in case of loss of coolant Training TMI accident was attributed to deficient personnel training Clear operating procedures ENGR302I Three Miles Island Near meltdown in March 28, 1979 Over 3 billion dollars in cleaning costs Two million people (within 50 miles radius) were exposed to low level radiation (no statistical way of determining how many will die from cancer) Nuclear Industry created a watchdog agency, Institute for Nuclear Power Operation (INPO) ENGR302I (Harrisburg, PA) Chernobyl, Ukraine April 26, 1986 explosion followed by fire in reactor#4 Fire extinguished by dropping 5000 tons of sand and boron. Eventually encased in 300,000 tons of concrete. Remaining Chernobyl reactors decommissioned in 1999. Why? RBK-1000 design unstable at low powers Poor training Inadequate containment Reactor was used as a research facility as well as power production ENGR302I Chernobyl – Consequences 8000 have already died. No estimate on the ultimate death toll from the accident 160,000 people were forced to leave their homes 1500 acres of surrounding forest died instantly; 5 million acres of prime farmland were contaminated; 20% of farmland and 15% of the Belarus forests cannot be used over 100 years. Rate of thyroid cancer in Ukrainian children have climbed 30fold. Higher rate of spontaneous abortion by Belarusian women Wild life is blooming. Field mice are undergoing evolutionary changes that took other species 10 million years. ENGR302I Nuclear Fusion ENGR302I Hydrogen Isotopes Abundance: Normal 99.8% Deuterium .015% Tritium .005% ENGR302I Fusion vs. Fission Fusion: D + T He + n + energy (DE=4x1011 BTU/kg deuterium) Fission U + n Kr + Ba +3n + energy (DE=7x1010 BTU/kg U-235) Coal (recall) C+O2 CO2+ energy (DE=3.3x104 BTU/kg coal) ENGR302I Fusion ENGR302I Fusion Reactions D+D @ 400 million oC He-3 + 3.3 MeV (79 MJ/g) D+T @ 45 million oC He-4 + 17.6 MeV (331 MJ/g) ENGR302I Breakeven Point Where plasma can be raised at sufficiently high temperature and particle density, and long enough so the rate of energy production exceeds the rate of energy required for sustained reaction. ENGR302I Challenges Ignition Very high temperatures to overcome repulsive forces of positively charged nuclei Confinement Very high pressures to increase probability of collision And for times long enough for producing energy more than that required for heating and compression (sustained reaction) ENGR302I Confinement No solid vessel Magnetic confinement Inertial confinement (laser) ENGR302I Magnetic Confinement ENGR302I