Absolute Dating - Cal State LA
advertisement

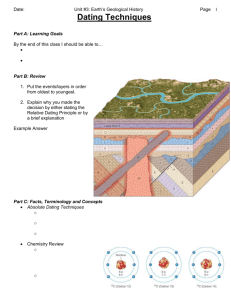
Absolute Dating Using Radioactivity Absolute Age dating was major advance in Historical Geology – More accurate reconstruction of geologic events Mostly based on radioactivity – Defined as spontaneous decay of one element to another – Pierre and Marie Curry -1903- decay produces heat What Are Absolute Dates The age of a rock, fossil, or geologic event expressed in units, such as years For example, your birthday . . . – You were born on a specific day, month and year Absolute age can be determined by radioactive decay The Atom Fundamental unit of matter Made up of components called subatomic particles – Protons (positive charge) – Neutron (no electrical charge) – Electron (negative charge Radioactive Decay – Stable Atoms An atom is generally stable if the number of protons equals the number of neutrons in the nucleus Atomic Number – An element’s identifying # – Equals # of protons in atom’s nucleus Mass Number – Equals # of protons and neutrons in an atom’s nucleus Radioactive Decay - Isotopes Variant of the same parent atom Differ in the # of neutrons Result in different mass # than parent For example: – Carbon-14 (C-14) – Types of carbon • C-12 (stable) • C-13 (unstable) • C-14 (unstable) Radioactive Decay The nucleus of an atom (decays) changes into a new element. The proton number (atomic number) changes 14 C 14N 6 7 Parent – unstable radioactive isotope Daughter – isotopes resulting from decay of parent Types of Radioactive Decay Alpha emission – Emission of 2 p+ and 2 n (α particle) – Mass # ↓ by 4 – Atomic # ↓ by 2 Beta emission – N emission of e- (β particle) – Mass # remains unchanged – Atomic # ↑ by 1 Electron Capture – An e- captured by p – e- + p = n – Mass # remains unchanged – Atomic # ↓ by 1 Radioactive Isotopes—Determine Decay Type Alpha emission – Emission of 2 p+ and 2 n (α particle) – Mass # ↓ by 4 – Atomic # ↓ by 2 Beta emission – N emission of e- (β particle) – Mass # remains unchanged – Atomic # ↑ by 1 Electron Capture – An e- captured by p – e- + p = n – Mass # remains unchanged – Atomic # ↓ by 1 U238 Radioactive Decay Series How Long Does Radioactive Decay Take? Half Life - time required for one-half of radioactive nuclei in a sample to decay – The half life of C-14 is 5,730 years 1. If start with 10,000 atoms of Carbon-14, how many will you have after 5 half-lives? 2. How old is sample? Carbon-14 Dating – The Carbon Cycle Carbon dating is common Only for young samples DECAY PROCESS FOR CARBON IS DIFFERENT FROM OTHER ISOTOPES! When Does the Clock Start?—Carbon Dating When a plant or animal dies, the clock starts. Organism dies No more C-14 intake C-14 begins to decay How the Carbon Clock Works There are two types of carbon used in the dating process – C-12 (stable does not decay) – C-14 (radioactive decays) When an organism is alive, it has the same ratio (C-12 to C-14) that is found in the atmosphere (1 trillion to 1) A living starfish has the same ratio as the atmosphere A fossilized starfish has a different ratio How the C-12:C-14 Ratio Works Amount of stable C-12 Amount of unstable C-14 Ratio Years dead # of half-lives 100 trillion 100 1-T to 1 0 0 100 trillion 50 2-T to 1 5,730 1 100 trillion 25 4-T to 1 11,460 2 100 trillion 12.5 8-T to 1 17,190 3 100 trillion 6 16-T to 1 22,920 4 100 trillion 3 32-T to 1 28,650 5 Carbon-14 can date elements up to approximately 100,000 years – Used to date very recent events – Important tool for anthropologists, archeologists and geologists Principles of Radioactive Dating Percentage of radioactive atoms that decay during one half-life is always the same (50 %) However, the actual # of atoms that decays continually decreases Comparing the ratio of parent to daughter yields the age of the sample Radioactive Isotopes Frequently used isotopes in Radiometric Dating Radioactive Isotopes U238 Radioactive Decay Series Magma Crystallization and Clock Begins Radiometric Dating Sources of Error – A closed system is required – Only fresh, non-weathered, unaltered or non-deformed rock samples should be used – For example, Metamorphism • Reheats samples • Sample measures younger than should be Can cross check age using other isotopes Isotope Used For Dating U-Pb & Th-Pb most common – Used for ancient samples—instrusives, lunar rocks, meteroites Rb-Sr used for oldest rocks K-Ar used for fine grained volcanic rocks – Ar is gas so sample must be fresh – Can also be used for metamorphic rocks Importance of Radiometric Dating A complex procedure that requires precise measurement Rocks from several localities have been dated at more than 3 billion years Confirms the idea that geologic time is immense Radiometric Dating Dating Sedimentary Strata How old are Dakota Sandstone, Mancos Shale and Mesaver?