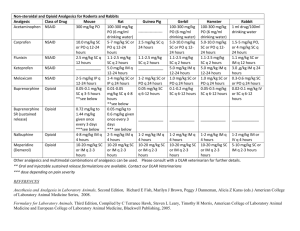
Using non-opioid pain medication first (FSMB model policies)
advertisement

Extended-Release and LongActing Opioid Analgesics Risk Evaluation and Mitigation Strategy (REMS) Central Appalachia Inter-professional Pain Education Collaborative (CAIPEC) Faculty Information Bios: Roberto Cardarelli, DO, MPH, FAAFP is Professor and Chief of Community Medicine at the University of Kentucky College of Medicine and is an active researcher in practice transformation and health services research. He serves as the Director of the Kentucky Ambulatory Network (KAN) with a history of research funding from NIH, Pfizer Medical Education, Meadows Foundation, American Academy of Family Physicians, Passport Health, and UK’s Center for Clinical and Translational Science. William Elder, PhD is a Professor and Director of Behavioral Science in the Department of Family and Community Medicine in Lexington, KY. As a clinical psychologist he sees patients with a full range of mental disorders. He leads or co led research and educational projects funded by HRSA, NIH, Pfizer Medical Education and others and he has served on multiple regional and national advisory panels for education and research. CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C 2 | © CO*RE 2014 Content Development/Planner/Reviewer Disclosures The following individuals disclose one or more relevant financial relationships: Roberto Cardarelli, DO, MPH Professor of Family % Community Medicine, University of Kentucky, Lexington, KY William Elder, PhD Professor of Family % Community Medicine, University of Kentucky, Lexington, KY Grant/research support consultant: Passport Health, UK CCTS, NIH, Pfizer, CDC, CMS, American Board of Family Medicine. Grant/research support consultant: HRSA, Pfizer, NIH CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C 3 | © CO*RE 2014 Central Appalachia Inter-professional Pain Education Collaborative (CAIPEC) Funded by an unrestricted grant from Pfizer Medical Education to develop and deliver a multi-faceted education program on chronic pain management to provider and healthcare teams in Central Appalachia. Future FREE events will include: • Webcasts • Community Roundtables • A clinic toolkit to help redesign how chronic pain management is delivered • Information on how to do QI projects and receiving Maintenance of Certification (MOC) credit CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Thank you for your participation today! Please take the pretest now – The pretest is part of the stapled paper handout you received Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics CAIPEC will also integrate the following Learning Objectives: • The Epidemiology of Chronic Pain • The Biopsychosocial Aspects of Chronic Pain • Chronic Pain History and Shared Decision Making • Examination and Diagnostic Testing in Patients with Chronic Pain • Non-Pharmacologic and Pharmacologic Treatment Options • Practice Enhancement in Managing People with Chronic Pain through a Teambased Approach Assessing Patients for Treatment with ER/LA Opioid Analgesic Therapy Module 1 Epidemiology Prevalence of chronic pain Not known Prevalence of significant $116 million pain, 2014 Financial cost of pain, 2014 $556 billion 2013 Behavioral Risk Factor Surveillance System C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative Epidemiology % Reporting Disability National Average 19.6 Kentucky 25.8 West Virginia 27.6 2013 Behavioral Risk Factor Surveillance System C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative Overdose Death, and Opioid Prescribing Rates State Age Adjusted Drug Overdose death Kilograms of prescription painkillers rate per 100,000 people (2008) sold, rates per 10,000 people (2010) Kentucky 17.9 West Virginia 25.8 9 9.4 C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative West Virginia • As the highest overdose rate nationally, WV’s rate is eight times higher than the lowest rate in North Dakota. • As of 2013, West Virginia’s number of drug overdose deaths - nearly 29 for every 100,000 residents – even surpassing those killed in auto accidents. • The number of overdose deaths in WV increased “6 fold” from 1999 to 2010 (mostly from prescriptions pills). • For more information about your state there are great resources available at the following site: http://www.rwjf.org/en/library/articles-and-news/2013/09/interactive--prescription-drugabuse.html Eyre, E., 2013. Report finds WV leads Nation in Drug Overdose Deaths, Journal of Emergency Medical Services, Retrieved from URL: http://www.jems.com/articles/2013/09/report-finds-west-virginia-leads-nation.html C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative Widespread Abuse and Misuse of Opioids In 2008, there were 14,800 prescription painkiller deaths http://www.cdc.gov/homeandrecreationalsafety/rxbrief/. Accessed Jan 2014. Drug Overdose Deaths by Major Drug Type, United States, 1999–2010 Opioids Heroin Cocaine Benzodiazepines 18,000 16,000 Number of Deaths 14,000 12,000 10,000 8,000 6,000 4,000 2,000 0 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Year CDC, National Center for Health Statistics, National Vital Statistics System, CDC Wonder. Updated with 2010 mortality data. 2010 Critical Vocabulary • Aberrant drug-related behavior: Conduct outside the boundaries of the agreed upon treatment plan • Abuse: Any use of an illegal drug, or a medication for a nonmedical purpose • Addiction: Impaired control over drug use, compulsive use, continued use despite harm, and/or craving • Diversion: The intentional transfer of a controlled substance from legitimate distribution and dispensing channels • Misuse: Use of a medication other than as directed or as indicated • Physical dependence: A state of biologic adaptation manifested by a withdrawal syndrome produced by decreasing blood levels of the drug • Tolerance: A state of physiologic adaptation in which exposure to a drug induces a diminution of one or more opioid effects over time Chou R, et al. J Pain. 2009;10(2):113-130. REMS Education Updated August 2014 On July 9, 2012, the FDA approved a Risk Evaluation and Mitigation Strategy (REMS) for extended-release (ER) and long-acting (LA) opioid medications Updated August, 2014 http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm163647.htm. Accessed Jan 2014. www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290. pdf. Accessed October 2014 What Drugs Are Covered by This REMS? Brand Name Products Generics • • • • • • • • • • • • • • • • • • • • • • Avinza® morphine sulfate ER capsules Butrans® buprenorphine transdermal system Dolophine® methadone hydrochloride tablets Duragesic® fentanyl transdermal system Embeda® morphine sulfate/naltrexone ER capsules* Exalgo® hydromorphone hydrochloride ER tablets Kadian® morphine sulfate ER capsules MethadoseTM methadone hydrochloride tablets MS Contin® morphine sulfate CR tablets Nucynta® ER tapentadol ER tablets Opana® ER oxymorphone hydrochloride ER tablets OxyContin® oxycodone hydrochloride CR tablets Palladone® hydromorphone hydrochloride ER capsules† ZohydroTM ER hydrocodone bitartrate ER capsules Targiniq® ER oxycodone HCl and naloxone HCl ER tablets Updated August 2014 Fentanyl ER transdermal systems Methadone hydrochloride tablets Methadone hydrochloride oral concentrate Methadone hydrochloride oral solution Morphine sulfate ER tablets Morphine sulfate ER capsules Oxycodone hydrochloride ER tablets *Not currently available due to voluntary recall (still approved) †No longer marketed (still approved) ER/LA Opioid Analgesics REMS. http://www.er-la-opioidrems.com/IwgUI/rems/products.action. Accessed Dec 2013. Risks of ER/LA Opioid Analgesics Updated August 2014 • Overdose with ER/LA formulations • Abuse by patient or household contacts – Especially adolescent children • Inadvertent exposure by household contacts • Misuse and addiction • Physical dependence and tolerance • Interactions with other medications and substances – Medication reconciliation • Neonatal opioid withdrawal syndrome with prolonged use during pregnancy • Financial (diverting drugs for illegal sale) Your Patient Complains of Pain: Where to Start • Consider source or etiology of pain • In most circumstances, use a non-opioid pain medication first FSMB Model Policy. http://www.fsmb.org/grpol_policydocs.html#2013. Accessed Dec 2013. 4a Describing Pain Acute Sudden onset, usually identifiable cause Last less than 1 month Chronic (our focus!) 3 months or greater, usually due to a chronic disease/condition; may have no obvious cause Prolonged physical, psychological impairments CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Biopsychosociology of Chronic Pain Pathology Process Normal pain abnormal pain CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Most people think of pain like Descartes Much more complex: Four physiological elements CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Element 1 Transduction Very complex: Nociceptors (afferent nerve endings) translate noxious stimuli into nociceptive impulses • 30 different nociceptor types • Silent nociceptors transmit only if inflammation present CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Element 2 Transmission Afferent nerve signals travel to dorsal root. Then project to thalamus, then to somatosensory cortexsensory aspects And frontal cortex and limbic systememotional responses CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Element 3: Modulation Process of dampening or amplifying pain related neural signals Primarily in dorsal horn Rich arrays of mu, kappa and delta opioid receptors 5HT and NE neurons Gate Control Endogenous ability of brain to increase or decrease pain transmission in the dorsal horn Element 4 Perception Subjective experience of pain Results from interaction of first three elements and psychology of individuals Brain determines if gate is open or closed Importance of psychological variables Gate can be controlled by pharmacological and psychological interventions CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Abnormal Pain • Not nociceptive pain • Pain that occurs in the context of nociceptive system that has been altered by tissue damage or other processes CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Abnormal Pain • Inflammatory pain • Acute injury causes tissue inflammation • Release of bradykinins and serotonin results in sensitization of peripheral neuroreceptors. Lower firing threshold • Silent receptors are recruited • If on-going inflammation (RA or CA) pain will persist • Inflammation may leave permanent alterations (OA) Central Sensitization Excessive or persistent nociceptive signals cause long term changes … • • • Wind up-amplification occurring when sufficient signal frequency alter C-Fibers • Nerve signals get trained to deliver pain signals better • Allodynia- abnormal response to light touch • Hypoalgesia • Primary: at the site of injury: thermal or mechanical • Secondary: central • Neuropathic pain • e.g., Nerves cut off from periphery, such as amputation became hyperactive • Dorsal horn changes altering sensory input: reorganization, enlargement of receptive fields, altered opioid sensitivity, enlarged sympathetic nerve terminals and abnormal temporal summation CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Somatic/Visceral Reflexes and Pain Somatic (ex. Muscles) afferent input can effect visceral organs and vice versa • Left arm pain during a heart attack • Right shoulder blade pain due to gallstones Be aware of these potential reflexes which may impact your workup and treatment plan CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C 2c “Learned” Pain CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Take home points Understanding these processes, their interactions and their alterations Appreciation of the multiple factors that influence pain Understanding how multiple types of interventions including cognitive behavioral therapies, mindfulness interventions, massage and PT may improve pain CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Clinical Interview: Getting Started • Complete history – Family history of substance abuse (does not preclude treatment with ER/LA opioid) – Family history of psychiatric disorders – Social history, including criminal record • Complete physical examination • Use a screening tool – ORT, SOAPP, DIRE Chou R, et al. J Pain. 2009;10(2):113-130. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm. Accessed Jan 2014. Clinical Interview: Description and Impact of Pain Description of current pain complaint • • • • • Location Intensity Quality Onset/Duration Patterns What causes or increases pain? Effects of pain on physical, emotional, and psychosocial function Patient’s pain and functional goals Heapy A, et al. Psychological and Behavioral Assessment. In: Raj's Practical Management of Pain. 4th ed. 2008;279-295. Zacharoff KL, et al. Managing Chronic Pain with Opioids in Primary Care. 2nd ed. Newton, MA: Inflexion, Inc., 2010. Clinical Interview: Pain Coping Strategies Pain Medications Past use Current use • • • Query state PDMP where available to confirm patient report Contact past providers and obtain prior medical records Conduct Urine Drug Test (UDT) Dosage • For opioid currently prescribed: opioid, dose, regimen, and duration – Important to determine if patient is opioid tolerant General Effectiveness Nonpharmacologic strategies and effectiveness Heapy A, Kerns RD. Psychological and Behavioral Assessment. In: Raj's Practical Management of Pain. 4th ed. 2008; 279-295. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. 2010. Risk Factors for Aberrant Drug-related Behaviors Family Hx Substance Abuse Personal Hx Substance Abuse Abuse Misuse Psychiatric Disorders Edlund MJ, et al. Pain. 2007;129(3):355-362. Chou R, et al. J Pain. 2009;10(2):113-130. Fishbain DA, et al. Pain Med. 2012;13(9):1212-1226. Younger Age Other Factors: • History of sexual abuse • Criminal record • Low reliability • Lack of social support • Smoking 5c Diagnostic Testing Dictated based on the H&P • Not needed (in most) cases- FP and FN consequences (procedures, further testing) • Red flag? (Foot drop, fecal incontinence, etc.) • Failed therapy, surgery is being considered • Rule in/out concerns- malignancy, fractures because of trauma CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C What Were They Thinking? • • • • Is this patient really in pain? Is he/she seeking opioids? Is he/she an abuser? Is he/she addicted? • Is the doctor taking my pain seriously? • Should I reveal my history? • Should I reveal my home life? Clinical Psychological Perspective Chronic pain should always be considered a mental disorder. First and foremost Somatoform Disorder Correct Diagnosis Somatic Symptom Disorder • Preoccupation with pain or abnormal reaction to pain • Examination out of proportion to disease or injury • Significant distress or dysfunction C A I P E C Central Appalachia Inter-Professional Pain Education Collaborative Key Psychological Factors • Temperament; Neurotic; Stoic • Coping style- active vs passive • Dysfunctional Coping, • History of trauma • Neurosurgical Outcomes Study • Pending settlements • Anxiety • Fearfulness including re-injury • Associated bracing • Cognitive Style • Catastrophic thinking • California Work Injury Studies • Depression +/• Cultural and social norms CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Social Side of Chronic Pain Assess social and family support Assess impact on work functioning Family members can: • • • • Provide assistance too much attention-secondary gain Be punishing when pain is expressed Families may need assistance in learning how to change their roles Provide simple intervention: E.g.. Encourage patient to distract from pain Work can: • • • The ratio of blue collar to white collar in terms of chronic pain related disability is at least 10 to 1. This is because of the nature of the work, Injuries affect job abilities, impacting self-esteem and identity Companies vary in how they will accommodate the injured employee -some punishing and some making job changes. Very few are willing to stick with someone with long standing injuries CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Psychosocial Evaluation Manage or refer when: • SI with or without plan • Lack of energy • Differentiated anxiety disorders: PTSD, Panic Disorder, OCD • Anhedonia • Unable to adjust to condition • Loss of appetite • Family dysfunction • Anger outbursts • Sleep issues; apnea insomnia CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Psychosocial Evaluation-Summary Questions to assess psychosocial aspects of pain Global How is the pain affecting your life? Emotional reactions What’s your mood generally like? Suicidal thoughts Do feel like giving up or hurting yourself? Cognitions, beliefs, coping with pain How do you cope with pain? Behavioral reactions What do you do if your pain flares up? Family functioning How do your family or others respond when you have pain? Social and occupational functioning How are work and social activities going? How are they impacted by your pain? CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Set Functional Goals Functional Status: • What’s a typical day like? • What’s the most active thing you do? • Do you ever stay in bed all day? • Do you get any exercise? • How have these things changed over the past weeks/months/years? • What would you (realistically) like to be able to do? CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Musculoskeletal Examination Do an overall assessment (i.e., neck, upper/lower extremities, trunk) and a focused palpitation exam at site of pain Note if deep or superficial pain Pay attention to patient’s response during exam Range of motion, assess functional restrictions Note the degree that exam can be performed before pain is elicited CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Neurological Examination Tailor exam based on pain complaint Assess motor and sensory systems • Sensory- light touch, pressure, thermal/ mechanical stimuli- allodynia (shouldn’t cause pain) • Hyperalgesia- severe pain from stimuli (ex. Pin-prick) that would be expected • Motor-weakness, ataxia, reduced endurance • Reflexes should be assessed CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Risk Management for Substance Dependency • All Biopsychosocial factors increase risk • Mental disorders (ADD, OCD, Bipolar disorder, schizophrenia) • Substance abuse in family or personally • Family link more significant factor in males CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Validated Questionnaires ORT Opioid Risk Tool SOAPP Screener & Opioid Assessment for Patients with Pain DIRE Diagnosis, Intractability, Risk, and Efficacy inventory STAR Screening Tool for Addiction Risk SISAP Screening Instrument for Substance Abuse Potential PDUQ Prescription Drug Use Questionnaire • No “gold standard” • Lack of rigorous testing Moore TM, et al. Pain Medicine. 2009;10(8):1426-1433. Opioid Risk Tool (ORT) Mark each box that applies 1. 2. Female Male Alcohol 1 3 Illegal drugs 2 3 Prescription drugs 4 4 Family Hx of substance abuse Administer On initial visit Prior to opioid therapy Personal Hx of substance abuse Alcohol 3 3 Illegal drugs 4 4 Prescription drugs 5 5 Scoring (risk) 3. Age between 16 & 45 yrs 1 1 0-3: low 4. Hx of preadolescent sexual abuse 3 0 5. Psychologic disease ADD, OCD, bipolar, schizophrenia 2 2 Depression 1 1 Scoring Totals: http://www.opioidrisk.com/node/2424. Accessed Jan 2014. Webster LR, et al. Pain Med. 2005;6:432-442. 4-7: moderate ≥ 8: high Concepts Comprising Medication Risk and Adherence Measures Evidence of Lying and Drug Use Fig. I . COMM concept mapping analysis and results conducted using the Concept System® software. Multi-Modal Approach Use or Restore SELF CARE Non-pharmacological therapies SELF EFFICACY Pharmacologic treatment Physical activity CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Non-pharmacologic Treatment Non-pharmacologic therapies that have proven beneficial for such patients include acupuncture, cognitive behavioral therapy, physical therapy, exercise therapy and therapeutic massage.2 2. Chou R, Qaseen A, Snow V, et al. Diagnosis and treatment of low back pain: a rjoint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147: 478-491 CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Psychosocial Intervention Psychosocial factors are better predictors of treatment outcomes than physical findings. Evaluation will identify factors related to poor outcomes (i.e. cognitive style, anxiety, passive attitudes towards rehabilitation) Fear avoidance beliefs, distress, somatization and pain catastrophizing place patients at the highest risk for poor outcomes. 1 Primary objectives in psychosocial intervention are: •Provide encouragement for overcoming demoralization •Help patient make connection between thoughts, feelings and behaviors •Teach patient coping strategies/techniques to adapt to pain and resultant problems 1. Elder, W., King, M., Dassow, P. and Macy, B. Managing Lower back pain: You may be doing too much, The Journal of Family Practice, 2009, 58 (4). Elder, W., King, M., Dassow, P. and Macy, B. Managing Lower back pain: You may be doing too much, The Journal of Family Practice, 2009, 58 (4). CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Non Pharmacological Options Massage Therapy: •Research shows massage: •restores pleasure body ( dopamine) •improves sleep, ( Serotonin) •improves ROM, •decreases pain ( Cortisol) Recent KAN pilot study with positive results for undifferentiated MT Elder, W. Chronic low back pain and primary health care, 2015 •No solid data about best techniques, optimal session length, or number of treatments so therapists need to use their clinical judgment. 45-60 min sessions (less if elderly or severely compromised) Once or twice a week (What client can afford and how therapist works) Client has undivided attention of LMT And will talk about stresses or problems getting them out of their system Many therapists include relaxation exercises during the session which clients can follow up with at home CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C © CO*RE 2014 53 | © CO*RE 2013 Non Pharmacological Options The LMT understands: “No Pain No Gain” does not work for Chronic pain Use inhibitory strokes to control the pain analyzing system and calm the hypersensitivity Giving the client some space from the pain Also asking the client to document where they are having pain each session using a body diagram and red pencil can show the client how pain changes over time •Establish connections to Licensed Massage Therapists At least 3-5 years experience more if available in your area Modalities like Swedish, Myofascial release, Neuromuscular Therapy, CranioSacral Therapy, have been used in massage trials with good outcomes Do they work with acute and or chronic pain? Get a session to feel their work. CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C © CO*RE 2014 54 | © CO*RE 2013 Clinical Interview: Conditions Suggestive of Abuse Hepatitis Cellulitis HIV Abuse? Cardiac/ Pulmonary disease Trauma, burns STD Chou R, et al. J Pain. 2009;10:113-130. Zacharoff KL, et al. Managing Chronic Pain with Opioids in Primary Care. 2nd ed. Newton, MA: Inflexion, Inc., 2010. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. 2010. Rationale for Urine Drug Testing (UDT) Patient Presentation • A 38-year-old divorced mother of three teenagers presents with complaints of lower back pain since an MVA 4 years ago • She describes the pain as constant, intense, and encompassing her whole lower back area. She relates that it is exacerbated by walking, bending, and lifting. The pain makes activities of daily life difficult • She would like to have her pain reduced to a tolerable level Patient Work-Up • Medical Hx – Prior physical therapy and medication have failed • Social Hx – Remote history of marijuana use – Once convicted of writing bad checks • Family Hx – Father abused alcohol – Recent break-up with an abusive boyfriend with a drug problem • Exam – While describing the severity of her pain and her limitations, she does not appear to be in pain – Lower back demonstrates tenderness with some wincing – Gait is normal Patient Case What is this patient’s risk of abuse, misuse, or other aberrant behavior? Low Medium High 1 2 3 4 5 6 7 ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ Patient Case What is the next step to refine the assessment for risk of aberrant behavior? ⃝ Obtain prior medical records ⃝ Use an assessment tool ⃝ Confirm UDT Special Considerations: Substance Abusers • Periodic UDT to confirm adherence • Regular re-evaluation to assure appropriateness of therapy – – – – Pain severity Functional ability Progress toward achieving therapeutic goals Adverse effects • Regular clinical assessment – Aberrant drug-related behaviors – Substance use – Psychological issues • Limit prescription quantities • Engage addiction or mental health experts Chou R, et al. J Pain. 2009;10(2):113-130. Special Considerations: Mental Health Issues • Increased risk of overdose • MH issues 3 times more prevalent in nonmedical users of opioids • SOAPP probes some factors – Antisocial behaviors/history – Psychiatric history – Psychosocial problems • Mental health will impact multiple aspects of treatment – Patient reliability – Aberrant drug-related behavior – Adherence Butler SF, et al. J Addict Med. 2009;3(2):66-73. Fischer B, et al. J Pain. 2012;13(11):1029-1044. Paulozzi LJ. J Safety Res. 2012;43(4):283-289. Referring High-Risk Patients Prescribers should Understand when to appropriately refer highrisk patients to pain management or addiction specialists Regularly check your state’s regulations for requirements http://www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed February 2014. Documentation • A risk/benefit evaluation – History – Physical exam – Diagnostic testing • Patient interactions • • • • Previous health records, including prescriptions, MRI, CT Patient permission to obtain records from other providers Provider communication with other providers Treatment plan, patient/provider agreement, informed consent (module 3) • Aberrant drug-related behavior Chou R, et al. J Pain. 2009;10(2):113-130. http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm163647.htm. Accessed Jan 2014. Module 1 Key Messages • ER/LA opioids can be effective for pain management • Benefit must be weighed against risk • Medical and behavioral factors influence risk of abuse or misuse • Patients should be regularly assessed • Documentation of assessments, patient interactions, treatment plans, aberrant drug-related behavior, and involvement of other providers is critical Initiating Therapy, Modifying Dosing, and Discontinuing Use of ER/LA Opioid Analgesics Module 2 Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics • CAIPEC will also integrate the following Learning Objectives: • The Epidemiology of Chronic Pain • The Biopsychosocial Aspects of Chronic Pain • Chronic Pain History and Shared Decision Making • Examination and Diagnostic Testing in Patients with Chronic Pain • Non-Pharmacologic and Pharmacologic Treatment Options • Practice Enhancement in Managing People with Chronic Pain through a Team-based Approach Federal & State Regulations Federal • Code of Federal Regulations, Title 21 Section 1306: rules governing the issuance & filling of prescriptions pursuant to section 309 of the Act (21 USC 829) – www.deadiversion.usdoj.gov/21cfr/cfr/2106cfrt.htm State • Database of state statutes, regulations, & policies for pain management – http://www.fsmb.org/pdf/grpol_pain_managem ent.pdf – www.medscape.com/resource/pain/opioid- policies • United States Code (USC) - Controlled Substances Act, Title 21, Section 829: prescriptions – www.deadiversion.usdoj.gov/21cfr/21usc/829.htm – http://statepolicyoptions.nga.org/policy_article/ database-state-statutes-regulations-and-otherofficial-governmental-policies Analgesic and Functional Goals • Decrease pain – Treat underlying cause where possible – Minimize medication use • Restore function – Physical, emotional, social • Correct secondary consequences of pain – Postural deficits, weakness, overuse – Maladaptive behavior, poor coping Patient Prescriber Agreements Informed Consent Communication process between patient and provider, including: • Potential risks and benefits – – – – – – Side effects (both short- and long-term) Tolerance and physical dependence Drug interactions and over-sedation Impaired motor skills Misuse, dependence, addiction, and overdose Evidence of benefit • Physician’s prescribing policies and expectations – Number and frequency of refills – Policy on early refills and replacement of lost or stolen medications • Specific reasons for changing or discontinuing opioid therapy, including violation of the treatment agreement www.fsmb.org. Accessed Jan 2014. Analgesic Selection Non-opioid approaches should be considered first • Address underlying condition • Non-opioid analgesia Short-acting opioids are probably safer than ER/LA for initial therapy • Shorter half-life • May have a lower risk of inadvertent overdose • Better for break-through pain Proposed benefits of long-acting opioids • More consistent control of pain • Improved adherence • Lower risk of addiction or abuse Chou R, et al. J Pain. 2009;10:113-130. Non-opioid medications CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C NSAIDS • 60 million Rx’s/yr • Clinical efficacy of equipotent doses is similar • Individual responses highly variable – especially toxicity • • • • • • • • cox-1 vs. cox-2 naprosyn may have greatest relative cardiovascular safety profile diclofenac - available as a topical patch for pain due to trauma and as a gel for treatment of painful joints sulindac – increased hepatotoxicity indomethacin - GI and central nervous system adverse effects may be more frequent or severe than with other NSAIDs ketorolac - risk of gastropathy is increased when use exceeds five days piroxicam – high GI toxicity celecoxib – no antiplatelet function. Increased CV risk above 200mg/day CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Utility of NSAIDS Variations in patient response Generally indicated in mild to moderate pain Mostly for pain of somatic origin although has a CNS effect as well Each trial should last a couple weeks May have an opioid sparing effect as adjunct Use at the maximum anti-inflammatory dose Protein bound – may interfere with other protein bound drugs (dilantin. coumadin) CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Tricyclic Antidepressants CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Utility in pain management • Most useful in neuropathic pain • May have synergy with opioids • None of the TCA’s carries • Switching TCA’s based on effect and/or side effects can be tried…but often frustrating any indication for pain management • Used frequently in variety of settings • Amitriptyline most widely studies • Anti-depressant effects may alleviate depression associated with chronic pain CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Mechanism of action Side effects • Generally unknown • Theories involve action on serotonin, norepinephrine receptors (TCAs with the greatest effect upon serotonin seem to have the greatest analgesic effect) • May potentiate endogenous opioid system • However, potent serotonin RI’s have no analgesic effect of their own • Can take weeks to work Anticholinergic (amitriptyline > nortriptyline) Also GI, CV, neurologic (esp. sedation – maybe a +’ve) Anticholinergic and CNS effects may diminish in days to weeks – “ride it out” CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Anticonvulsants CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Utility in pain management Can be very effective, particularly in neuropathic pain Wide variation in use among pain specialists, except with carbamazepine for trigeminal neuralgia Gabapentin is frequently a first choice as levels do not need monitoring CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Mechanism of action Theories include membrane stabilization (phenytoin), inhibition of repeated neuronal discharges (carbamazepine), GABA inhibition enhancement (valproic acid, clonazepam), GABA mimetics (gabapentin, pregabalin). CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C **Benzodiazepines** Suitable choice when anxiety complicates pain management (especially cancer patients) and when non-pharmacological treatments unavailable Clonazepam particularly useful in neuropathic pain (GABA potentiation) Drawbacks well known • Addictive potential is significant • Potentiates sedation and respiratory depression CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Muscle relaxants & Antispasmodics Painful muscle spasm, myoclonic jerks can accompany a variety of pain conditions (and Opioids) • toxicity of morphine Mechanism of action may reflect their sedative effects more than direct muscle effect Commonly used – cyclobenzaprine, baclofen, methocarbamol CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C SSRI/SNRI •Often tried when TCA side effects limit utility •May be treating depression – not an uncommon consequence of life with chronic pain •Venlafaxine has been shown to be similar to imipramine in one study of painful neuropathy •Duloxetine approved for diabetic peripheral neuropathy •Depression is probably undertreated in chronic pain patients in general (cancer and non-cancer pts) CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C When to Consider a Trial of an ER/LA Opioid Pain is severe Patient failed to adequately respond to non-opioid & non-drug interventions Pain has an adverse impact on function or quality of life Potential therapeutic benefits outweigh potential harms Consider referral to pain or addiction specialist when risks outweigh benefits http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/UCM367726.htm. Accessed April 2014. Chou R, et al. J Pain. 2009;10:113-130. When to Not Consider a Trial of an ER/LA Opioid Pain is acute Condition is amenable to nonopioid or non-drug interventions Patient is at high risk of aberrant drugrelated behaviors Presence of contraindications Chou R, et al. J Pain. 2009;10:113-130. Initiating & Titrating ER/LA Opioid Analgesics: Opioid-Naïve Patients Drug/Dose Monitor Titrate • For naïve? • Drug PI • Respiratory Depression • 24-72 hours • Efficacy • Tolerability • Adverse Effects • Minimum interval Drug PI Chou R, et al. J Pain. 2009;10:113-130. When Analgesia is Inadequate Reassess/manage underlying condition Address behaviors that aggravate pain Apply other non-opioid medications Escalate opioid dose as a trial Consider an ER/LA opioid (if using IR opioid) Consider opioid rotation Converting From Immediate Release to ER/LA Opioids • Safety is primary • Before conversion to a long-acting opioid, use immediate release preparations to titrate to the appropriate 24 hour dose • Different approaches A. Use opioid equianalgesic dose table (EDT, conversion table) to switch B. Use cross-titration • Slowly decrease old opioid • Slowly increase new opioid • May take weeks to achieve proper dosing Switching ER/LA Opioids: Rotation Dissatisfaction • • • • • • AE Poor efficacy Drug interactions Route of administration Change in clinical status Financial or drug-availability Weigh • • • • Age Clinical state Comorbidities Other medications Other Drug Fine PG, et al. J Pain Symptom Manage. 2009;38:418-425. • • • • • Drug sensitivities Convenience Improved adherence Financial Best dose!?! You have decided to switch ER/LA medications. At what dose should the second ER/LA opioid be initiated? ⃝ Equivalent dose, from the ED table ⃝ 25–50% reduction of equivalent dose ⃝ 75% reduction of equivalent dose ⃝ Initiate as new treatment after wash-out period Choosing a New Dose Calculate equianalgesic dose of new opioid from EDT Reduce calculated equianalgesic dose • Generally: 25–50% reduction • Methadone: 75–90% reduction • Use clinical judgment ~50% reduction if patient ~25% reduction if patient • Receives a high dose of current opioid • Elderly or medically frail • Is staying on current opioid but switching to a different administration route Fine PG, et al. J Pain Symptom Manage. 2009;38:418-425. Guidelines for Opioid Rotation If switching to methadone: • Reduce calculated equianalgesic dose by 75–90% • For patients on very high opioid doses, be cautious converting to methadone ≥ 100 mg/d ‒ Consider inpatient monitoring, including serial EKG If switching to transdermal formulation: • Fentanyl: use equianalgesic dose ratios in the PI • Buprenorphine: follow instructions in the PI Fine PG, et al. J Pain Symptom Manage. 2009;38:418-425. Guidelines for Opioid Rotation Titrate new opioid dose Frequently assess: Analgesia AEs Withdrawal symptoms Break Through Pain • Use a short-acting, immediate release preparation at 5–15% of total daily opioid dose • If oral transmucosal fentanyl is used for BTP, always begin at lowest dose • NEVER use ER/LA opioids for BTP Fine PG, et al. J Pain Symptom Manage. 2009;38:418-425. Opioid Rotation: Summary Values from EDT Frequently assess initial response †If Patient opioid values “Solve” for X Titrate dose of new opioid to optimize outcomes Calculate supplemental rescue dose used for titration at 5–15% of total daily dose‡ switching to methadone, reduce dose by 75–90% fentanyl used as rescue, begin at lowest dose irrespective of baseline opioid ‡If oral transmucosal Fine PG, et al. J Pain Symptom Manage. 2009;38:418-425. Always reduce dose+ Improper Opioid Rotation Can Be Fatal • Nearly 15,000 people die every year as a result of overdoses involving prescription painkillers • 5 of the 15 drugs most frequently named in FDA-reported fatal outcomes were opioids • Why? – – – – – – Polysubstance abuse Nonmedical use and misuse Prescriber’s competence Proliferation of inconsistent guidelines for opioid rotation Use of equianalgesic tables as conversion tables Limitations inherent in the equianalgesic dose tables Webster LR, et al. Pain Med. 2012;13(4):562-570. ER/LA Opioid-Induced Respiratory Depression Chief hazard of opioids • May lead to respiratory arrest & death • Greatest risk after initiation or dose increase • Alcohol, sedatives, hypnotics, etc Reduced urge to breathe & decreased respiration rate Instruct patients/ family • Symptoms • Shallow breathing • Call 911 • CO2 retention can • Dangerous exacerbate opioid sedating effects polypharmacy Chou R, et al. J Pain. 2009;10:113-130. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 2012. www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafety InformationforPatientsandProviders/UCM311290.pdf Opioid Exit Strategy – Possible Paths Patient’s behavior consistent with drug addiction • Refer for addiction management or comanagement Patient unable or unwilling to cooperate with outpatient taper No apparent addiction problem Patient able to cooperate with officebased taper • • Provide limited and closely monitored prescriptions while referring to more intensive services • Taper gradually over 1-2 months Implement nonopioid pain management (psychosocial support, CBT, PT, non-opioid analgesics) CBT, cognitive behavioral therapy; PT, physical therapy. Katz, N. Patient Level Opioid Risk Management: A Supplement to the PainEDU.org Manual. Newton, MA: Inflexxion, Inc; 2007. Webster LR, Dove B. Avoiding Opioid Abuse While Managing Pain: A Guideline for Practitioners. 1st Edition. North Branch, MN: Sunrise Press; 2007. Taper Dose When Discontinuing Avoid withdrawal symptoms in opioid dependent patients Optimal setting • Outpatient in absence of unstable medical or psychiatric conditions or unsafe patterns of behavior • Higher risk patients may need a more structured setting When aberrant drug-related behaviors continue, may need • Close monitoring and enforced tapering or • Discontinued prescription and referral to detox or • Agonist therapy May use a range of approaches • Slow…10% dose reduction/week • Rapid…25–50% reduction every few days Chou R, et al. J Pain. 2009;10:113-130. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy Taper Dose When Discontinuing Factors that influence the reduction rate: • Reason for discontinuation • Medical and psychiatric comorbidities • Initial weaning rate – Faster at high doses (eg, > 200 mg/d morphine equivalent) – Slower at low doses (eg, 60-80 mg/d morphine equivalent) • Monitor withdrawal symptoms After taper: • Continue to treat pain with non-opioids • Treat psychiatric disorders • Assess for and treat addiction-related aberrant behaviors Chou R, et al. J Pain. 2009;10:113-130. Shared Decision Making (SDM) • Collaborative process for doctors and patients, to use best evidence available while also addressing irrational choices, weighted according to patient values • Respects autonomy • Good for long term decisions in chronic illness involving multiple visits Physicians trained in a SDM approach to chronic pain report: • self-reported change in care of patients with chronic pain (P =.01) • greater overall physician satisfaction (P=.002) • more appropriate use of time (P =.02) • self-reported completion of treatment contracts (P =.01) • Self-reported rates of methadone prescribing (P =.05) Charles, C. Soc Sci & Med. 1997 Joosten, et al. Psychother Psychosom 2008;77:219–226 Sullivan et al, J Gen Intern Med. 2006 Apr; 21(4): 360–362.) CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Shared Decision Making Involves: • Asking patient about preferred role in decision-making. • Asking about values and clarifying values • Setting goals for care and outcomes • Negotiating in context of patient’s preferences. Shared Decision Making Principles: •Empathy and validation: “you’ve been through a lot.” •Partner: “Together I want to work on…” •Set goals: “Change will take some time.” •Promote self-efficacy/optimism: “positive changes.” •Buy-in: “A re you ready to take this on?” •Explain when hit resistance CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Utilize Shared-Decision Making Uncontrolled chronic pain is found more often in patients who • Are passive • Catastrophize • Perceive an external locus of control Counteract these by requiring the patient to make decisions and set goals with you. CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Decisions That Can’t Be Shared • Policies and procedures for prescriptions, refills, and monitoring • If and when to discontinue a medication when risks or harms outweigh benefits ↓ ↓ • Approach should still be patient-centered – Emphasize risks/benefits to patient More info and an online course at: https://www.sgim.org/File%20Library/SGIM/Resource%20Library/Meeting%20Handouts/SGIM/2011/WD06handout.pdf http://www.coperems.org/ CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Module 2 Key Messages • Best analgesic choice depends on patient and condition − Non-opioid analgesia − Immediate-release opioid − ER/LA opioid • Rotation/conversion is not an exact science but protocols give guidance • ER/LA are not for breakthrough pain • Respiratory depression is rare but serious • Opioids should be discontinued by tapering Managing Therapy with ER/LA Opioid Analgesics Module 3 Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics • CAIPEC will also integrate the following Learning Objectives: • The Epidemiology of Chronic Pain • The Biopsychosocial Aspects of Chronic Pain • Chronic Pain History and Shared Decision Making • Examination and Diagnostic Testing in Patients with Chronic Pain • Non-Pharmacologic and Pharmacologic Treatment Options • Practice Enhancement in Managing People with Chronic Pain through a Team-based Approach Analgesic and Functional Goals • Decrease pain – Treat underlying cause where possible – Minimize medication use • Restore function – Physical, emotional, social • Correct secondary consequences of pain – Postural deficits, weakness, overuse – Maladaptive behavior, poor coping Pain Assessment • Current pain – Numeric rating scale – Visual analog scale – Faces scale • Pain history Patients sometimes assume that you don’t believe their pain complaints • PQRS Measure #131 They may exaggerate pain scores Pain Management Scales Pain Management scales used for assessing pain include: •Pain Assessment and Documentation Tool (PADT) Progress Note Pain Assessment and Documentation Tool (PADT™) PDI: No Disability 0__. 1__. 2__. 3__. 4__. 5__. 6__. 7 __. 8__. 9__. 10__. Worst Disability •Pain Disability Index (PDI) •Universal Pain Assessment tool (Wong-Baker FACES pain rating scale + 0-10 numeric pain rating) CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Self-Reflection on Patient Management How often do you use quantitative scales for pain, functional status, and adverse events? Never Sometimes Always 1 2 3 4 5 6 7 Pain ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ Function ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ AEs ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ ⃝ CDC HRQOL Healthy Days Measure 1. Would you say that, in general, your health is: 2. Now thinking about your PHYSICAL HEALTH, which includes physical illness and injury, for how many days during the past 30 days was your physical health not good? 3. Now thinking about your MENTAL HEALTH, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good? 4. During the past 30 days, for about how many days did poor physical or mental health keep you from doing your usual activities, such as self-care, work, or recreation? http://www.cdc.gov/hrqol/hrqol14_measure.htm#1. Accessed Jan 2014. Side Effect Rating Scale: FIBSER Frequency, Intensity, and Burden of Side Effects Rating Frequency Intensity Impact • % time present • None always • 0-6 scale • Severity • None intolerable • 0-6 scale • Degree of interference • None nonfunctional • 0-6 scale Wisniewski SR, et al. J Psychiatr Pract. 2006;12(2):71-79. http://www.medscape.org/viewarticle/752781. Accessed Jan 2014. Patient Prescriber Agreements Informed Consent Communication process between patient and provider, including: • Potential risks and benefits – – – – – – Side effects (both short- and long-term) Tolerance and physical dependence Drug interactions and over-sedation Impaired motor skills Misuse, dependence, addiction, and overdose Evidence of benefit • Physician’s prescribing policies and expectations – Number and frequency of refills – Policy on early refills and replacement of lost or stolen medications • Specific reasons for changing or discontinuing opioid therapy, including violation of the treatment agreement www.fsmb.org. Accessed Jan 2014. Patient Prescriber Agreements Written Opioid Treatment Agreement Treatment Goals Roles Responsibilities Pain Function Monitoring Grounds for Continuation Medication safeguards Progress towards goals Visits Decision Tolerable AEs Adherence to agreement ______________________________, Patient ______________________________, Provider Cheatle MD, et al. J Pain Symptom Manage. 2012;44(1):105-116. _______Date _______Date Rationale for Urine Drug Testing (UDT) Prior to Therapy During Therapy • Prior drug use • Other drug use • Adherence • Legal requirement • Grounds for referral • Frequency per provider UDT Stages SCREENING CONFIRMATION • Immunoassay • Drug class • Qualitative (+/-) • Lab or POC • GC/MS or LC/MS • Specific, definitive • Quantitative • Lab SAMHSA. Clinical Drug Testing in Primary Care. Technical Assistance Publication (TAP) 32. HHS Publication No.(SMA) 12-4668. Rockville, MD: SAMHSA, 2012. Specific Windows of Drug Detection Drug in Urine Time Amphetamines ≤2d THC – Single use – Chronic use 1-3 d ≤ 30 d Benzoylecgonine after cocaine use 2-4 d Opiates (morphine, codeine) 2-3 d Methadone ≤3d ≤6d – EDDP (methadone metabolite) Benzodiazepines (depending on drug and dose) Moeller KE, et al. Mayo Clin Proc. 2008;83(1):66-76. Days to weeks Interpretation of Confirmed Results Positive Result Demonstrates recent use • Most drugs have detection times of 1-3 d • Chronic use of lipidsoluble drugs: test positive for ≥ 1 week Does not diagnose Can’t determine • Drug addiction, physical dependence, or impairment • Metabolism can alter results • Exposure time, dose, or frequency of use Negative Result Does not diagnose diversion May be due to maladaptive drug-taking behavior • More complex than presence or absence of a drug in urine • Bingeing, running out early • Other factors: eg, cessation of insurance, financial difficulties Milone MC. J Med Toxicol. 2012;8(4):408-416. Interpretation of UDT Results • Use UDT results in conjunction with other clinical information • Investigate unexpected results Discuss with the lab Discuss with the patient A positive result is probably …. a positive result. • Document results, interpretation, and action • May necessitate closer monitoring and/or referral to a specialist Screening for Substance Abuse • Pain Assessment and Documentation Tool (PADT) – 17 Y/N questions – Plus pain and function sections • Current Opioid Misuse Measure (COMM) – 17 questions – 5 item Likert scale Passik SD, et al. Clin Ther. 2004;26(4):552-561. https://www.nhms.org/node/128 Accessed Jan 2014. Butler SF, et al. Pain. 2007;130(1-2):144-156. http://www.painedu.org/. Accessed Jan 2014. Recognizing Addiction • DSM-5 Criteria for Substance Use Disorder1 ≥ 2 items Dx – Tolerance – Withdrawal – Taken more/longer than intended ≥ 6 items severe case – Desire/unsuccessful efforts to quit use – Great deal of time taken by activities involved in use – Use despite knowledge of problems associated with use – Important activities given up because of use – Recurrent use resulting in a failure to fulfill important role obligations – Recurrent use resulting in physically hazardous behavior (eg, driving) – Continued use despite recurrent social problems associated with use – Craving for the substance • Pain Medicine Questionnaire2 (PMQ, 26 self-report items) • Screener and Opioid Assessment for Patients with Pain3 (SOAPP, 24 self-report items) 1. 2. 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition. Arlington,VA: American Psychiatric Publishing(2013). Adams LL, et al. J Pain Symptom Manage. 2004;27(5):440-459. Butler SF, et al. Pain. 2004;112(1-2):65-75. Case • You have started a 49-year-old married mother of 2 grown children on a short-acting opioid for failed back syndrome. She got little relief from the first dose you prescribed and little relief from 3 months of a higher dose – Your staff points out that she has requested early refills twice • Her son calls about his mother’s treatment • He reports that she is uncommunicative, spending more time in bed and on the sofa, and not leaving the house for social events and church as was her custom. This information conflicts with the patient’s reports to you that she is – Functioning as before – Having lots of pain • A random UDT is positive for your opioid and a benzodiazepine • At the next visit the patient reports that she is – Functioning as before – Having lots of pain • Her physical exam is unchanged • You decide to speak candidly with her about her care Confidence in Patient Management What conclusion(s) can you make on the basis of the UDT? (choose all that apply) She is taking the opioid as directed She may be abusing other drugs She is not taking the opioid as directed She may be addicted Common Adverse Events Nausea/Vomiting • Tend to self-resolve • Switch opioid • Antiemetic Sedation • Tends to self-resolve • Decrease dose Constipation Pruritus • Laxative • Bowel stimulant • Switch opioid • Tends to self-resolve • Antihistamines • Switch opioid Many patients report being allergic to pain medication, especially opioids Swegle JM, et al. Am Fam Physician. 2006;74(8):1347-1354. Benyamin R, et al. Pain Physician. 2008;11(2 Suppl):S105-S120. Universal Precautions for Prescribing Controlled Substances 1. 2. 3. 4. 5. Make a Diagnosis with Appropriate Differential Psychological Assessment Including Risk of Addictive Disorders Informed Consent Treatment Agreement Pre- and Post-Intervention Assessment of Pain Level and Function 6. Appropriate Trial of Opioid Therapy +/– Adjunctive Medication 7. Reassessment of Pain Score and Level of Function 8. Regularly Assess the “Five A’s” of Pain Medicine • Analgesia • Adverse effects • Activity • Aberrant behavior • Affect 9. Periodically Review Pain Diagnosis and Comorbid Conditions, including Addictive Disorders 10. Documentation Adapted from Gourlay DL, et al. Pain Med. 2005;6(2):107-112. Taking a Team-Based Approach You need to translate this new/updated knowledge into clinical practice Pain management is a team approach Your staff within the clinic and •Other professional colleagues outside the clinic We will focus on how to re-design the way your practice manages patients with chronic pain CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Taking a Team-Based Approach The general categories in a chronic pain workflow: Clinic policy and registration processes • Clinic policies that patients sign at check in Self-administered assessments for new and established chronic pain patients • Selected self assessments patient complete while they wait Clinical check-in and staff assessments • Nursing assessments and activities in the exam room CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Taking a Team-Based Approach The general categories in a chronic pain workflow (continued): Clinical assessment • Provider H&P Post clinical activities • UDS, Controlled medication agreements, etc. Post visit activities • Referrals, diagnostic orders, follow-up on results/consults, etc. CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Step 1: Team-Based Approach This must become a priority for your clinic All members (front office to back staff) must be part of the re-design process Educate one another about pain management (use CAIPEC webcasts or other online sources) Educate as a team how to do the Plan-Do-Study- Act (PDSA) quality improvement approach CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Step 2: Team-Based Approach Now that everyone knows why it is important and on board to improve things: •Use the PDSA to identify something you want to improve on (ex. Ensuring 100% of CP patients have contracts/agreement, etc.). •Define an ultimate goal for this specific project •Find an area (start small) that the clinic process needs to improve on •Map out using workflows on how that process currently happens and what needs to change •Identify who does what in the new workflow CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Step 3: Team-Based Approach The Plan is in place, time to execute (“Do”). You need a way to monitor the progress and collect some data Tweak as you go, this is normal Pay attention and document what is working and not working CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Step 4: Team-Based Approach Once you feel things have progressed and have collected enough information, “Study” it: How much better are you doing over your baseline? What have you and the team learned CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Step 5: Team-Based Approach Once you studied your results, you now “Act” for the next PDSA cycle: What can we do better? What didn’t work? What do you now add to our chronic pain management process? Prescreening tools? New standardized visit forms or templates? All these steps are done with the clinic team The PDSA cycle is a repetitive processes until you reach your ultimate goal CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Team-Based Approach Developing workflows that are “live” documents that everyone has and adheres to helps to sustain interventions • They make people accountable for their part of the process CAIPEC provides a toolkit with sample documents, forms, workflows, etc. that you can adapt for your clinic CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C Workflow model for clinic practice CCentral Appalachia A Inter-Professional I P Pain Education E Collaborative C 136 | © CO*RE 2013 Module 3 Key Messages • Set specific analgesic and functional goals • Use a PPA as a framework • Document discussions, patient commitments, actions, results • Refer patients for addiction and abuse treatment as needed • Identify and manage adverse events Counseling Patients and Caregivers about the Safe Use of ER/LA Opioid Analgesics Module 4 Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics Patient Counseling Document: The DOs of ER/LA Opioids • READ the medication guide – – – – Take your medicine exactly as prescribed Store your medicine in a safe place away from children Flush unused medicine down the toilet Seek help if you do not understand something • REPORT side effects – – Call your health care provider Report to the FDA at 1-800-FDA-1088 • CALL 911 or your local emergency service right away if – You take too much medicine – You have trouble breathing or shortness of breath – A child has taken this medicine • TALK to your health care provider – – – If the medication does not control your pain About side effects About all your medicines: Rx, OTC, vitamins, and supplements http://www.er-la-opioidrems.com/IwgUI/rems/pcd.action. Accessed Jan 2014. Patient Counseling Document: The DON’Ts of ER/LA Opioids DON’T give your medicine to others DON’T take medicine unless it was prescribed for you DON’T stop taking your medicine without talking to your health care provider DON’T break, chew, crush, dissolve, or inject your medicine DON’T drink alcohol while taking this medicine http://www.er-la-opioidrems.com/IwgUI/rems/pcd.action. Accessed Jan 2014. Reflection on Clinical Practice How do you use the ER/LA opioid prescribing information when counseling a patient with chronic pain? (choose all that apply) I don’t generally refer to it I use it to discuss dosing I use it to discuss side effects and warnings I use to structure the patient discussion I give a copy to patients http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed Jan 2014. Product-Specific Information • Drug Prescribing Information documents include critical information – Indications, usage – Dosage forms and strengths, administration – Contraindications, warnings, precautions – Common adverse reactions, drug interactions – Specific populations – Counseling information, medication guide • Easily available: http://www.accessdata.fda.gov/scripts/cder/dru gsatfda/index.cfm One of your patients travels a lot and has an irregular lifestyle. She is scrupulously adherent; her testimony is supported by UDTs. Airline seats and lack of exercise have exacerbated her back pain and she has developed tolerance to oxycodone. What is your recommendation? ⃝ Increase her oxycodone dose ⃝ Convert to a transdermal formulation ⃝ Taper her oxycodone and seek a non-opioid analgesic FDA Labeling Supplement Boxed Warning Updated August 2014 • TRADENAME exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk before prescribing, and monitor regularly for development of these behaviors or conditions. (5.1) • Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Instruct patients to swallow TRADENAME (formulation) whole to avoid exposure to a potentially fatal dose of (active opioid). (5.2) • Accidental consumption of TRADENAME, especially in children, can result in fatal overdose of (active opioid). (5.2) • For patients who require opioid therapy while pregnant, be aware that infants may require treatment for neonatal opioid withdrawal syndrome. Prolonged use during pregnancy can result in life-threatening neonatal opioid withdrawal syndrome. (5.3) For products with an interaction with alcohol, also include the following: • Instruct patients not to consume alcohol or any products containing alcohol while taking TRADENAME because co-ingestion can result in fatal plasma (active opioid) levels. (5.4) www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm367697.pdf. Accessed October 2014. Special Warnings for Patients: Labeling Supplement Updated August 2014 • Never break, chew, or crush an oral ER/LA tablet/capsule, or cut or tear patches prior to use – May lead to rapid release of ER/LA opioid causing overdose and death – Applesauce or feeding tube may be appropriate (consult PI) • Use of other CNS depressants with ER/LA opioids can cause overdose and death – Sedative-hypnotics and anxiolytics – Alcohol – Illegal drugs • Use other CNS depressants, including other opioids, under the instruction of their prescriber • Talk to your provider about your medical history, as many functions can be impacted by ER/LA opioids • Do not abruptly stop or reduce the ER/LA opioid dose • After you stop taking your drug, flush any unused tablets down the toilet www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm367697.pdf. Accessed October 2014. Medication Guide • Each PI contains detailed patient instructions about dosing, custody, precautions, disposal, etc • Lay language, should be shared with patients verbally and in printed form • Patient literacy and/or language barriers must be identified and addressed http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed October 2014. Review All Medications: What Other Drugs Might Impact Treatment? Alcohol Other Opioid Source PGP Inhibitors Opioids CYP Inhibitors Inducers Antiarrhythmics MAO Inhibitors BZD Specific Drug Interactions Alcohol* PGP Inhibitors Morphine sulfate ER (Avinza) Morphine sulfate ER (Kadian) Product Benzodiazepines MAO Inhibitors Class IA + III Antiarrhythmics Oxycodone HCl (OxyContin) Oxymorphone HCl (Opana ER) CYP 450 Inhibitors/ Inducers Morphine sulfate CR (MS Contin) Morphine sulfate ER-Naltrexone (Embeda) CYP 3A4 Inhibitors/ Inducers Hydromorphone HCl (Exalgo) Methadone (Dolophine) Tapentadol (Nucynta ER) Fentanyl Transdermal (Duragesic) Buprenorphine Transdermal (Butrans) FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsa ndProviders/UCM311290.pdf. Accessed January 2014. PGP: P-glycoprotein MAO: monoamine oxidase *Including medications containing alcohol Diversion in the Home Own the Prescriptions • Note how many pills in each prescription bottle or packet • Take inventory of all prescription drugs in your home • Track refills for all household members • Keep meds in a safe place, not the bathroom cabinet Know Their Weaknesses • Teens: adventure • Elderly: drug naïveté Discard Expired or Unused Meds Adapted from The Partnership at DrugFree.org. Rx Abuse: Not in My House. http://notinmyhouse.drugfree.org/steps.aspx . Accessed January 2014. Public Awareness Campaign When to Discontinue Opioids • Severe unmanageable adverse effects • Serious or persistent nonadherence to the treatment plan • Illegal or unsafe behaviors • Misuse suggestive of addiction to prescribed medication • Lack of effectiveness • Patient preference • Decreased level of pain in stable patients • Goals of treatment are not met http://www.healthquality.va.gov/cot/. Accessed Jan 2014. Tapering Opioids: General Considerations • Individualize; faster or slower tapering may be warranted • Complete evaluation prior to initiation of the taper – Current treatment plan – Psychological conditions – Other relevant factors should be completed • Clear written and verbal instructions should be given to patients and their families to minimize withdrawal symptoms http://www.healthquality.va.gov/cot/. Accessed Jan 2014. Tapering Opioids: Patients to Refer • High risk to engage in aberrant behaviors (eg, parasuicidal acts; dealing/selling medications; severe impulse control disorders) • Complicated withdrawal symptoms • Opioid addiction • Refer to an addiction or pain specialist! http://www.healthquality.va.gov/cot/. Accessed Jan 2014. Tapering Opioids: Patient Considerations • Do not treat withdrawal symptoms with opioids or benzodiazepines after discontinuing opioids • Consider tapering opioids in patients who have received regularly scheduled opioids at greater than the recommended starting doses for more than a few days • Non-daily, as-needed opioids do not usually need tapering • Patient-specific factors – Risk of precipitating withdrawal – Level of anxiety about discontinuing – Duration of opioid therapy (longer use slower taper) – Medical and psychological comorbidities – Clinical need for rapid taper http://www.healthquality.va.gov/cot/. Accessed Jan 2014. agencymeddirectors.wa.gov/Files/OpioidGdline.pdf. Accessed Jan 2014. Tapering Opioids: Specific Considerations • Taper by 20–50% per week for patients who are not addicted • A patient needs 20% of the previous day’s dose to prevent withdrawal symptoms • Consider adjuvant agents such as antidepressants to manage irritability and sleep disturbance, or antiepileptics for neuropathic pain • Patient on fentanyl should be rotated to a different opioid, either long-acting morphine or methadone – Once the patient is converted, the same guidelines will apply http://www.healthquality.va.gov/cot/. Accessed Jan 2014. agencymeddirectors.wa.gov/Files/OpioidGdline.pdf. Accessed Jan 2014. How should patients dispose of expired or unused opioids? Trash Community drug collection Flush down toilet Mix with kitty litter and put into trash First Choice: Community Drug Take-Back • National Prescription Drug Take-Back Day: “Got Drugs?” • More than 5000 sites participate • Check with local government for day/location www.fda.gov/consumer. www.awarerx.org. Accessed Jan 2014. http://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. Accessed January 2014. FDA Drug Disposal Guidelines • • • • • Follow the prescription drug labeling Community drug take-back programs Container: scratch out all identifying information on the label Do not give your medicine to friends When in doubt, talk to your pharmacist Dispose of unused ER/LA opioids by flushing down the toilet http://www.fda.gov/forconsumers/consumerupdates/ucm101653.htm. Accessed Jan 2014. www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Module 4 Key Messages • Use a Patient Counseling Document to communicate • Refer to drug-specific Prescribing Information – Includes a Medication Guide for patients – Proper disposal of drugs • An exit strategy is critical – Know when to discontinue opioids – Know how to taper opioids – Know when to refer to a specialist Anyone ready for a break? General Drug Information for ER/LA Opioid Analgesic Products Module 5 Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics Drug Information Common to ER/LA Opioid Analgesics Limitations of usage • Not for as-needed analgesia • Not for mild pain or pain not expected to persist for an extended duration • Not for use in treating acute pain FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Oct 2014. Drug Information Common to ER/LA Opioids Key Instructions Updated August 2014 Reserve for use in patients for whom alternative options are ineffective, not tolerated, or inadequate Titrate a dose that provides adequate analgesia and minimizes adverse reactions Times required to reach steady-state plasma concentrations are product specific (Refer to PI for titration interval) Continually reevaluate patient to assess pain control and adverse effects Consult drug PI for dosage reduction with hepatic or renal impairment FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 08/2014 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Oct 2014. A 32-year-old patient who suffered multiple fractures in a motor vehicle accident at age 16 has chronic pain. He is on metoprolol, NSAIDs, gabapentin, and alprazolam but needs increased pain relief. What is the best next step? ⃝ Taper the alprazolam before ER/LA opioid initiation ⃝ Initiate an ER/LA opioid but monitor for respiratory depression ⃝ Begin with a medication reconciliation to avoid drug-drug interactions ⃝ Try a different non-opioid analgesic first ER/LA Opioids: Transdermal Dosage • Application – Rotate location of application – Prepare skin Clip (don’t shave) hair; wash only with water • Safety – Strenuous exertion or external heat exposure may lead to increased absorption and possible overdose – Monitor patients with fever for signs/symptoms of increased opioid exposure – Handle and dispose appropriately Avoid exposure of caregivers/children – Do not cut, damage, chew or swallow – Products with metal foil backings are not safe for MRIs FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions CNS Depressants ER/LA Opioid Analgesics Concurrent Use • Sedatives • Hypnotics • General anesthetics • Antiemetics • Phenothiazines • Other tranquilizers • Alcohol ↑ Risk of: • Respiratory depression • Hypotension • Profound sedation • Coma Reduce the initial dose of one or both agents FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions Partial agonists and mixed agonist/antagonist analgesics ER/LA Opioid Analgesics Concurrent Use • Buprenorphine • Pentazocine • Nalbuphine • Butorphanol • May reduce analgesic effect • Precipitate withdrawal symptoms Avoid concurrent use FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions Antiarrhythmic Agents • Methadone • Buprenorphine Concurrent Use Antiarrhythmic Agents such as: • Class IA – Quinidine – Procainamide – Disopyramide • Class III – Sotalol – Amiodarone – Dofetilide • May cause QTc prolongation/ arrhythmia Avoid use of these opioids in patients with long QT Syndrome or those taking antiarrhythmic medications FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions CYP Inhibitors/Inducers may impact blood levels of ER/LA opioids CYP Inhibitors Opioid Levels CYP Inducers Opioid Levels !! Consult product-specific information !! FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions Drug Potential Risk • Alcohol; medications with alcohol • • Monoamine oxidase inhibitors (MAOIs) Diuretics Skeletal muscle relaxants Anticholinergic medications • Some ER opioid formulations may rapidly release opioid when exposed to alcohol (“dose dump”) Some drug levels may increase without dose dumping when exposed to alcohol See individual PI • Using opioids with MAOIs may increase respiratory depression May cause serotonin syndrome • Opioids can reduce diuretic efficacy by inducing ADH • Opioids may enhance the neuromuscular blocking action of skeletal muscle relaxants and increase respiratory depression • Concurrent use increases the risk of urinary retention and severe constipation/paralytic ileus FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Drug Interactions Illicit Drugs • A retrospective study of 21,746 patients treated with opioids for chronic pain • Urine samples analyzed to determine co-occurrence of illicit drugs – – – – 13% + for THCA (tetrahydrocannabinol) 4.6% + for cocaine 1.1% + for methamphetamine Of those using marijuana, 14% were also using cocaine or methamphetamine • Patients using illicit drugs and prescribed opioids are at risk for reactions from the abused substances and from drugdrug interactions Pesce A, et al. Pain Physician. 2010;13:283-287. ER/LA Opioid Analgesics Tolerance to sedating and respiratory depressant effects of opioids is critical to the safe use of certain products/strengths/doses • Products used ONLY in opioid-tolerant patients – Transdermal fentanyl – Hydromorphone HCl ER • For other products, patients must be opioid tolerant before using – Certain strengths – Certain daily doses – Refer to individual PI FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. ER/LA Opioid Analgesics Relative Potency to Morphine • Intended as a general guide • Follow conversion instructions in individual PI • Incomplete cross-tolerance and inter-patient variability require conservative dosing when converting from 1 opioid to another – Halve the calculated comparable dose; titrate new opioid as needed FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311 290.pdf. Accessed Jan 2014. Module 5 Key Messages • Prescribers should be knowledgeable about general characteristics, class adverse effects, and drug interactions for ER/LA opioid analgesic products – This information provides the foundation for assimilation of product-specific information and the safe/effective use of ER/LA opioid analgesics Specific Drug Information for ER/LA Opioid Analgesic Products Module 6 Learning Objectives • Appropriately assess patients for the treatment of pain with ER/LA opioid analgesics, including analyzing risks versus potential benefits • Assess patient’s risk of abuse, including substance use and psychiatric history • Identify state and federal regulations on opioid prescribing • Incorporate strategies to effectively initiate therapy, modify dosing or discontinue use of ER/LA opioid analgesics in patients with pain • Manage ongoing therapy with ER/LA opioid analgesics • Incorporate effective counseling for patients and caregivers about the safe use of ER/LA opioid analgesics • Discuss general and product-specific drug information related to ER/LA opioid analgesics Informed Treatment Decisions Consider product characteristics that align with patient needs Drug substance • Formulation • Strength Dosing interval Key instructions • Titration • Limitations of usage • Product conversion information Specific drug interactions Product-specific safety concerns Use in opioid-tolerant patients Potency relative to oral morphine DailyMed: www.dailymed.nlm.nih.gov Drugs@FDA: www.fda.gov/drugsatfda A Look at Different ER/LA Opioid Analgesic Products • Compare and contrast – Relative potency of ER/LA opioid agents – Dosing frequency – Use based on opioid exposure Oral – Specific drug interactions • Morphine sulfate (4) • Specific characteristics • Oxycodone • Oxymorphone • Hydromorphone • Methadone • Tapentadol Transdermal • Buprenorphine • Fentanyl Relative Potency to Oral Morphine Important to consult PIs for use of opioids as first agents and for conversion/rotation procedures Morphine 1.0 Oxycodone 2:1 Oxymorphone 3:1 Hydromorphone Transdermal 5:1 Fentanyl > 100:1 Dose Ratio (Oral Morphine to Other Agents) Methadone • Varies contingent on prior opioid exposure Not Established • Buprenorphine • Tapentadol FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Dosing Frequency Product Every 8 or 12 hours Methadone (Dolophine) Morphine sulfate CR (MS Contin) Every 12 hours Tapentadol (Nucynta ER) Oxymorphone HCl (Opana ER) Oxycodone HCl (OxyContin) Once/Day or Every 12 hours Morphine sulfate ER (Kadian) Morphine sulfate ER-Naltrexone (Embeda) Once/Day Morphine sulfate ER (Avinza) Hydromorphone HCl ER (Exalgo) Fentanyl Transdermal (Duragesic) Every 3 Days Every 7 Days Buprenorphine Transdermal (Butrans) FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Morphine Sulfate ER-Naltrexone: Embeda 20 mg/0.8 mg, 30 mg/1.2 mg, 50 mg/2 mg, 60 mg/2.4 mg, 80 mg/3.2 mg, and 100 mg/4 mg Capsules Dosing • Interval • • • Instructions • • • Specific Drug Interactions • Once/day or every 12 hours Initial dose as first opioid: 20 mg/0.8 mg Titrate using a minimum of 3-day intervals Swallow capsules whole (do not chew, crush, or dissolve) Crushing or chewing will release morphine, possibly resulting in fatal overdose, and naltrexone, possibly resulting in withdrawal symptoms May open capsule and sprinkle pellets on applesauce for patients who can reliably swallow without chewing, use immediately Alcoholic beverages or medications containing alcohol may result in the rapid release and absorption of a potentially fatal dose of morphine PGP inhibitors (eg, quinidine) may increase the absorption/exposure of morphine sulfate by about 2-fold Use in Opioid • Tolerant Patients 100 mg/4 mg capsule is for use in opioid-tolerant patients only Product-Specific • Safety Concerns None FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Morphine Sulfate ER-Naltrexone: Embeda 20 mg/0.8 mg, 30 mg/1.2 mg, 50 mg/2 mg, 60 mg/2.4 mg, 80 mg/3.2 mg, and 100 mg/4 mg Capsules Dosing • Interval • • • Instructions • • • Specific Drug Interactions • Once/day or every 12 hours Initial dose as first opioid: 20 mg/0.8 mg Titrate using a minimum of 3-day intervals Swallow capsules whole (do not chew, crush, or dissolve) Crushing or chewing will release morphine, possibly resulting in fatal overdose, and naltrexone, possibly resulting in withdrawal symptoms May open capsule and sprinkle pellets on applesauce for patients who can reliably swallow without chewing, use immediately Alcoholic beverages or medications containing alcohol may result in the rapid release and absorption of a potentially fatal dose of morphine PGP inhibitors (eg, quinidine) may increase the absorption/exposure of morphine sulfate by about 2-fold Use in Opioid • Tolerant Patients 100 mg/4 mg capsule is for use in opioid-tolerant patients only Product-Specific • Safety Concerns None FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Oxycodone Hydrochloride CR: OxyContin 10, 15, 20, 30, 40, 60, and 80 mg Tablets Dosing Interval Instructions • Every 12 hours • • • • Opioid-naïve patients: initiate treatment with 10 mg every 12 hours Titrate using a minimum of 1 to 2 day intervals Hepatic impairment: start with one third to one half the usual dosage Renal impairment (creatinine clearance < 60 mL/min): start with one half the usual dosage Consider use of other analgesics in patients who have difficulty swallowing or have underlying GI disorders that may predispose them to obstruction. Swallow tablets whole (do not chew, crush, or dissolve) Take one tablet at a time, with enough water to ensure complete swallowing immediately after placing in the mouth • • • Specific Drug Interactions • • CYP3A4 inhibitors may increase oxycodone exposure CYP3A4 inducers may decrease oxycodone exposure Use in Opioid Tolerant Patients • Single dose greater than 40 mg or total daily dose greater than 80 mg are for use in opioid-tolerant patients only • • Choking, gagging, regurgitation, tablets stuck in the throat, difficulty swallowing the tablet Contraindicated in patients with gastrointestinal obstruction • ~ 2:1 oral morphine to oxycodone oral dose ratio Product-Specific Safety Concerns Relative Potency to Oral Morphine FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Oxycodone Hydrochloride CR: OxyContin 10, 15, 20, 30, 40, 60, and 80 mg Tablets Dosing Interval Instructions • Every 12 hours • • • • Opioid-naïve patients: initiate treatment with 10 mg every 12 hours Titrate using a minimum of 1 to 2 day intervals Hepatic impairment: start with one third to one half the usual dosage Renal impairment (creatinine clearance < 60 mL/min): start with one half the usual dosage Consider use of other analgesics in patients who have difficulty swallowing or have underlying GI disorders that may predispose them to obstruction. Swallow tablets whole (do not chew, crush, or dissolve) Take one tablet at a time, with enough water to ensure complete swallowing immediately after placing in the mouth • • • Specific Drug Interactions • • CYP3A4 inhibitors may increase oxycodone exposure CYP3A4 inducers may decrease oxycodone exposure Use in Opioid Tolerant Patients • Single dose greater than 40 mg or total daily dose greater than 80 mg are for use in opioid-tolerant patients only • • Choking, gagging, regurgitation, tablets stuck in the throat, difficulty swallowing the tablet Contraindicated in patients with gastrointestinal obstruction • ~ 2:1 oral morphine to oxycodone oral dose ratio Product-Specific Safety Concerns Relative Potency to Oral Morphine FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Methadone Hydrochloride: Dolophine 5 and 10 mg Tablets Dosing Interval Instructions • Every 8 to 12 hours • • Initial dose in opioid non-tolerant patients: 2.5 to 10 mg Conversion of opioid-tolerant patients using equianalgesic tables can result in overdose and death. Use low doses according to the table in the full prescribing information High inter-patient variability in absorption, metabolism, and relative analgesic potency Opioid detoxification or maintenance treatment shall only be provided in a federally certified opioid (addiction) treatment program (CFR, Title 42, Sec 8) • • • • Pharmacokinetic drug-drug interactions with methadone are complex – CYP 450 inducers may decrease methadone levels – CYP 450 inhibitors may increase methadone levels – Anti-retroviral agents have mixed effects on methadone levels Potentially arrhythmogenic agents may increase risk for QTc prolongation and torsade de pointes Benzodiazepines may increase respiratory depression Use in Opioid Tolerant Patients • Refer to full prescribing information Product-Specific Safety Concerns • • • • QTc prolongation and torsade de pointe Peak respiratory depression occurs later and persists longer than analgesic effect Clearance may increase during pregnancy False positive urine drug screens possible Relative Potency to Oral Morphine • Varies depending on patient’s prior opioid experience Specific Drug Interactions • FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Methadone Hydrochloride: Dolophine 5 and 10 mg Tablets Dosing Interval Instructions • Every 8 to 12 hours • • Initial dose in opioid non-tolerant patients: 2.5 to 10 mg Conversion of opioid-tolerant patients using equianalgesic tables can result in overdose and death. Use low doses according to the table in the full prescribing information High inter-patient variability in absorption, metabolism, and relative analgesic potency Opioid detoxification or maintenance treatment shall only be provided in a federally certified opioid (addiction) treatment program (CFR, Title 42, Sec 8) • • • • Pharmacokinetic drug-drug interactions with methadone are complex – CYP 450 inducers may decrease methadone levels – CYP 450 inhibitors may increase methadone levels – Anti-retroviral agents have mixed effects on methadone levels Potentially arrhythmogenic agents may increase risk for QTc prolongation and torsade de pointes Benzodiazepines may increase respiratory depression Use in Opioid Tolerant Patients • Refer to full prescribing information Product-Specific Safety Concerns • • • • QTc prolongation and torsade de pointe Peak respiratory depression occurs later and persists longer than analgesic effect Clearance may increase during pregnancy False positive urine drug screens possible Relative Potency to Oral Morphine • Varies depending on patient’s prior opioid experience Specific Drug Interactions • FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed Jan 2014. Tapentadol ER: Nucynta ER 50, 100, 150, 200, and 250 mg Tablets Dosing Interval Instructions • Every 12 hours • • • • • Use 50 mg every 12 hours as initial dose in opioid nontolerant patients Titrate by 50 mg increments using a minimum of 3-day intervals Maximum total daily dose is 500 mg Swallow tablets whole (do not chew, crush, or dissolve) Take one tablet at a time and with enough water to ensure complete swallowing immediately after placing in the mouth Dose once daily in moderate hepatic impairment with 100 mg per day maximum Avoid use in severe hepatic and renal impairment • • • • Alcoholic beverages or medications containing alcohol may result in the rapid release and absorption of a potentially fatal dose of tapentadol Contraindicated in patients taking MAOIs Use in Opioid Tolerant Patients • No product-specific considerations Product-Specific Safety Concerns • • Risk of serotonin syndrome Angioedema Relative Potency to Oral Morphine • Equipotency to oral morphine has not been established Specific Drug Interactions FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Tapentadol ER: Nucynta ER 50, 100, 150, 200, and 250 mg Tablets Dosing Interval Instructions • Every 12 hours • • • • • Use 50 mg every 12 hours as initial dose in opioid nontolerant patients Titrate by 50 mg increments using a minimum of 3-day intervals Maximum total daily dose is 500 mg Swallow tablets whole (do not chew, crush, or dissolve) Take one tablet at a time and with enough water to ensure complete swallowing immediately after placing in the mouth Dose once daily in moderate hepatic impairment with 100 mg per day maximum Avoid use in severe hepatic and renal impairment • • • • Alcoholic beverages or medications containing alcohol may result in the rapid release and absorption of a potentially fatal dose of tapentadol Contraindicated in patients taking MAOIs Use in Opioid Tolerant Patients • No product-specific considerations Product-Specific Safety Concerns • • Risk of serotonin syndrome Angioedema Relative Potency to Oral Morphine • Equipotency to oral morphine has not been established Specific Drug Interactions FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Fentanyl: Duragesic (continued) 12, 25, 50, 75, and 100 mcg/hr Transdermal System Dosing Interval • One transdermal system every 3 days (72 hours) Specific Drug Interactions • • CYP3A4 inhibitors may increase fentanyl exposure CYP3A4 inducers may decrease fentanyl exposure Use in Opioid Tolerant Patients • All doses of fentanyl are indicated for use in opioid-tolerant patients only • Accidental exposure due to secondary exposure to unwashed/unclothed application site Increased drug exposure with increased core body temperature or fever Bradycardia Application site skin reactions Product-Specific Safety Concerns • • • Relative Potency to Oral Morphine • See individual product information for conversion recommendations from prior opioid FDA. Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics. 04/2013 www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290 .pdf. Accessed Jan 2014. Module 6 Key Messages • Safe prescribing of ER/LA opioid analgesics requires knowledge of product-specific information • Product factors to consider when formulating individualized treatment plans: Safety Dosing Formulation Tolerance Potency Drug interactions Key Instructions Thank you for participating! • Please take the posttest • Please fill out the evaluation