Lecture Packet#2
advertisement

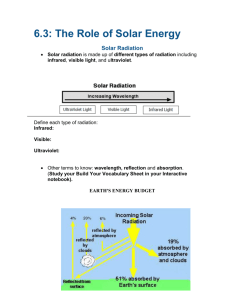
Warming the Earth and the Atmosphere Chapter 2 This chapter discusses: • Temp and heat transfer – Conduction, convection, radiation • Solar radiation, earth energy balance • The seasons – Local variations Temperature and Heat Transfer Temperature is the quantity that tells us how hot or cold something is relative to some set standard value. • Air is made up of billions of atoms and molecules, moving in all directions, spinning and bumping around. They don’t all move at same speed. – Energy associated with this motion is called kinetic energy, the energy of motion. – Temperature of air is the measure of its average kinetic energy – Temperature is a measure of the average speed of the atoms and molecules, where higher temperatures correspond to faster average speeds. – Absolute zero – at this temp the atoms and molecules would posses a minimum amount of energy and theoretically no thermal motion Temperature Scales • • • • • Kelvin Scale - a temp scale begins at absolute zero (“no motion”) Fahrenheit Scale – assigned 32 as the number where water freezes and 212 where water boils. 180 equal divisions called degrees Celsius Scale – Zero on this scale assigned to the temperature at which pure water freezes and 100 to temp where pure water boils. Divided into 100 equal degrees C=5/9(F-32) K=C+273 Figure 1: Comparison of the Kelvin, Celsius, and Fahrenheit scales Latent Heat Change of State (Phase Change)- changes from gas…liquid…solid (ice) • The heat energy required to change a substance (water), from one state to another is called latent heat. (Why do we say latent?) • Examine a drop of water – evaporating – faster moving molecules escape most easily and the average motion of all the molecules left behind decreases as each additional molecule evaporates. Slower motion suggest a lower water temperature. Evaporation is, therefore, a cooling process. • The energy lost by the liquid water during the evaporation can be thought of as carried away by, and “locked up” within the water vapor molecules. The energy is in a “stored” or “hidden” condition and is called latent heat. • The heat is latent (hidden) in that the temperature of the substance changing from liquid to vapor is still the same. Figure 3: Heat energy absorbed and released This latent heat energy will reappear as sensible heat (that we can feel and measure with a thermometer) when the vapor condenses back into liquid water. Condensation (opposite of evaporation) is a warming process. Latent heat is an important source of atmospheric energy! Water vapor rising into the air cools and becomes liquid water and ice particles…this process releases heat into the environment Figure 3: Every time a cloud forms, it warms the atmosphere. Inside this developing thunderstorm a vast amount of stored heat energy (latent heat) is given up to the air, as invisible water vapor becomes countless billions of water droplets and ice crystals. In fact, for the duration of this storm alone, more heat energy is released inside this cloud than is unleashed by a small nuclear bomb. Conduction and Convection The transfer of heat from the hot end of the metal pin to the cool end by molecular contact is called conduction. Molecules in the end of the pin absorb energy from the flame and vibrate faster than those farther away from flame, energy is eventually transferred from molecule to molecule to hand. The transfer of heat by the mass movement of a fluid (such as water and air) is called convection. Convection happens naturally in the atmosphere. (I.e. Thermals – rising bubbles of air.) Note: In our atmosphere, air that rises expands and cools air that sinks is compressed and warmed! Figure 5: The development of a thermal. A thermal is a rising bubble of air that carries heat energy upward by convection Radiation The energy transferred from the sun to your face on a warm day is called radiant energy or radiation. The sunlight travels through the air with little effect on the air itself. The energy travels in the form of waves that release energy when they are absorbed by an object. These are called electromagnetic waves because they have magnetic and electrical properties. micrometer Figure 6: Radiation characterized according to wavelength. As the wavelength decreases, the energy carried per wave increases. Note different wave lengths, micrometer is 10-6 Photons Think of radiation as streams of particles,or photons, that are discrete packets of energy. UV photons carry more energy than a photon of visible light. UV photons produce sunburns, penetrate skin, can cause cancer. Figure 8: The sun's electromagnetic spectrum and some of the descriptive names of each region. The numbers underneath the curve approximate the percent of energy the sun radiates in various regions. Solar radiation and Earth radiation Figure 8: The hotter sun not only radiates more energy than that of the cooler earth (the area under the curve), but it also radiates the majority of its energy at much shorter wavelengths. (The area under the curves is equal to the total energy emitted, and the scales for the two curves differ by a factor of 100,000.) Solar radiation is shortwave radiation. Earth radiation is longwave radiation Balancing Act – Absorption, Emission, and Equilibrium If the earth and all things on it are continually radiating energy, why doesn't everything get progressively colder? • The rate at which something radiates and absorbs energy depends strongly on its surface characteristics, such as color, texture, moisture and temperature. • Blackbody – an object that is a perfect absorber (it absorbs all the radiation that strikes it) and a perfect emitter (emits the maximum radiation possible at its given temperature). Does not have to be black in color. Earths surface is nearly 100% efficient and thus behaves like a blackbody • Radiative equilibrium temperature – Average temp at which the rate of absorption of solar radiation equals the rate of emission of infrared earth radiation. Selective Absorbers and the Atmospheric Greenhouse Effect Many selective absorbers in the environment. • Snow, good absorber of IR radiation, but poor absorber of sunlight. Good emitter of IR energy. At night snow emits more IR energy than it absorbs (loss of IR radiation helps cause the air above the ground to become very cold). • Others are water vapor and CO2 Figure 9: Absorption of radiation by gases in the atmosphere. The shaded area represents the percent of radiation absorbed. The strongest absorbers of infrared radiation are water vapor and carbon dioxide. Water vapor and carbon dioxide (CO2) are strong absorbers of IR Radiation and poor absorbers of visible solar radiation. They radiate a portion of the IR energy toward the ground and act as act as an insulating layer around the earth, keeping part of the radiation emitted by the earth from escaping into space. Insolation Figure 10a: Sunlight warms the earth's surface only during the day, whereas the surface constantly emits infrared radiation upward. Without water vapor, CO2, and other greenhouse gases, the earth's surface would constantly emit infrared radiation (IR); incoming energy from the sun would be equal to outgoing IR energy from the earth's surface. Without the greenhouse effect, the earth's average surface temperature would be -18°C (0°F) . Figure 10b: With greenhouse gases, the earth's surface receives energy from the sun and infrared energy from its atmosphere. Incoming energy still equals outgoing energy, but the added IR energy from the greenhouse gases raises the earth's average surface temperature about 33°C, to a comfortable 15°C (59°F). If convection were to suddenly stop - the average Earth temp would rise about 18F Figure 11: Air in the lower atmosphere is heated from below. Sunlight warms the ground, and the air above is warmed by conduction, convection, and radiation. Further warming occurs during condensation as latent heat is given up to the air inside the cloud. Most absorption takes place near the surface – lower atmosphere is mainly heated from below. At sunrise and sunset, when the white beam of sunlight must pass through the a thick portion of the atmosphere, scattering by air molecules removes the blue light, leaving the longer wavelengths of red, orange and yellow to pass on through A brilliant red sunset produced by the process of scattering On the average, of all the solar energy that reaches the earth's atmosphere annually, about 30 percent (30/100) is reflected and scattered back to space, giving the earth and its atmosphere an albedo of 30 percent. Of the remaining solar energy, about 19 percent is absorbed by the atmosphere and clouds, and 51 percent is absorbed at the surface. The earth-atmosphere energy balance. Numbers represent approximations based on surface observations and satellite data. While the actual value of each process may vary by several percent, it is the relative size of the numbers that is important. Aurora – caused by charged particles from the sun interacting with the atmosphere. Solar wind collides with atmospheric gases. Gases get “excited” and emit light aurora borealis in NH and aurora australis in SH The stream of charged particles from the sun - called the solar wind distorts the earth's magnetic field into a teardrop shape known as the magnetosphere. When an excited atom, ion, or molecule deexcites, it can emit visible light. The electron in its normal orbit becomes excited by a charged particle... ...and jumps into a higher energy level. When the electron returns to its normal orbit, it emits a photon of light. Aurora Belt • The aurora belt (solid red line) represents the region where you would most likely observe the aurora on a clear night. (The numbers represent the average number of nights per year on which you might see an aurora if the sky were clear.) The flag MN denotes the magnetic north pole, whereas the flag NP denotes the geographic north pole. Why do we have seasons? • Earth has an elliptical path around the sun that takes a little over 365 days • One spin on its own axis in 24 hours • Average distance from earth to sun is 93 million miles • Elliptical path takes us closer to sun in January than it does in July – Say what? • Seasons are regulated by sun angle and the number of daylight hours The elliptical path (highly exaggerated) of the earth about the sun brings the earth slightly closer to the sun in January than in July. Sunlight that strikes a surface at an angle is spread over a larger area than sunlight that strikes the surface directly. Oblique sun rays deliver less energy (are less intense) to a surface than direct sun rays. As the earth revolves about the sun, it is tilted on its axis by an angle of 23.5°. The earth's axis always points to the same area in space (as viewed from a distant star). Thus, in June, when the Northern Hemisphere is tipped toward the sun, more direct sunlight and long hours of daylight cause warmer weather than in December, when the Northern Hemisphere is tipped away from the sun. (Diagram, or course, is not to scale.) Land of the Midnight Sun. A series of exposures of the sun taken before, during, and after midnight in northern Alaska during July. Note: The sun never sets from Mar 20 – Sept 22. Equinox Autumnal (fall) Equinox – Sept 22 – Sun is directly above the equator. Except for poles, days and nights throughout the world are Equal length. After this in the NH there are fewer hours of daylight and The noon sun is slightly lower in the sky. Winter Solstice – Dec 21 - The shortest day of the year, and the astronomical beginning of winter in NH. Summer Solstice – June 21 – Sun is highest in sky in N.H. and directly over 23 ½ N (Tropic of Cancer) Vernal (spring) Equinox – March 20, once again the noonday sun is Shining directly on the equator, days and nights are of equal length, And at the North pole the sun rises above the horizon after 6 long dark Months. During the Northern Hemisphere summer, sunlight that reaches the earth's surface in far northern latitudes has passed through a thicker layer of absorbing, scattering, and reflecting atmosphere than sunlight that reaches the earth's surface farther south. Sunlight is lost through both the thickness of the pure atmosphere and by impurities in the atmosphere. As the sun's rays become more oblique, these effects become more pronounced. The average annual incoming solar radiation (red line) absorbed by the earth and the atmosphere along with the average annual infrared radiation (blue line) emitted by the earth and the atmosphere. The changing position of the sun, as observed in middle latitudes in the Northern Hemisphere. In areas where small temperature changes can cause major changes in soil moisture, sparse vegetation on the south-facing slopes will often contrast with lush vegetation on the north-facing slopes.