Measurement Test Review
advertisement

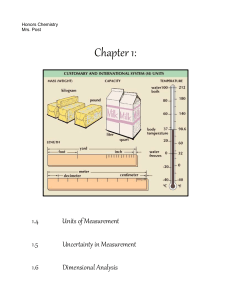
Measurement and Matter Review Sig Figs 4. For the following measurements, indicate how many significant figures there are: a) 34 g ___ b) 504 L ___ c) 3.4 x 103g _____ d) 23.45 mg ___ e) 101 km ___ f) 7.0 x 10-5mm___ g) 5040 L ___ h) 0.0190 mm ___ i) 20 mg ___ j) 20. km ___ k) 20.0 g ___ l) 0.02 L ___ 6. Perform all calculations and express your answer with the appropriate sig figs & units: a) 67 cm x 55 cm = 3700 cm2 b) 4.29 m x 9.8 m = ________ c) 870 mm x 430 mm = ________ d) 0.034 g/L x 8.8 L = ________ e) 1.405 m x 6.39 m = ________ f) 3.56 cm x 2.45 cm x 0.83 cm = _________ g) 45.9 mi ÷ 1.50 hr = _________ h) (4.6x107m) x (7.32x10-3m) = __________ i) (4.6x107g) / (7.32x10-3cm3) = __________ j) 3.56 g ÷ (2.6 cm x 4.3 cm x 7.8 cm) = _____ 7) Perform all calculations & give your answers with the appropriate units and sig figs. a) 6.74 g + 2.1 g = ______ b) 1200 kg + 286 kg = ______ c) 13.531 s + 4.1 s = _____ d) 7800 cm - 2 cm = ______ e) 784.326 m - 2 m = 782m f) 2.54 g - 0.000034 g = ______ 8) Show your work for all of the following calculations. Remember units and sig figs. a) A box is 235.8 cm by 45.2 cm by 7.9 cm. Its volume (V = l x w x h) is: __________ c) A 5627 g brick measures 5.60 cm x 4.51 cm x 24.71 cm. Its density (D=m/V) is: ______ 11) An object with a mass of 7.5g raises the level of water in a graduated cylinder from 25.1mL to 30.1mL. What is the density of the object? 12) The density of aluminum is 2.7g/mL. What is the volume of 8.1g? 14) A 2.75 kg sample of a substance occupies a volume of 250.0 cm3. Find its density in g/cm3 15) A rectangular block of lead (Pb) measures 20.0 mm X 30.0 mm X 45.0 mm. If the density of Pb is 11.34 g/cm3, calculate the mass of the block. Classification of Matter 1. Identify the following as representing either a physical change (P) or a chemical change (C) a. Wood turns to ash when burned d. Iron melts b. Frying an egg e. Alcohol evaporates on a countertop c. Water freezes f. Grains fermenting 2. Identify the following properties as either PHYSICAL (P) or CHEMICAL (C) and EXTENSIVE (E) or INTENSIVE (I): P/C E/I P/C E/I a. A liquid has a volume of 50.2 ml h. Acids and bases are corrosive b. Ethanol is flammable i. Sugar is soluble in water c. Copper has a density of 8.92 g/cm3 j. Silver reacts with nitric acid d. The iron sample has a mass of 26.4g k. Water evaporates to water vapor e. Sodium has a silver color l. f. m. Coke has a pH of about 3 Water has a boiling point of 100°C g. Salt solutions conduct electricity 3. Matching: a. Homogeneous Mixture b. Compound c. Element d. Heterogeneous Mixture Methane is combustible n. Glycerol is a viscous liquid i. Matter composed of a simple substance that cannot be broken down any further ii. Matter composed of more than one substance that looks different throughout iii. Pure substance created chemically that has different properties than the substances that form it iv. Matter composed of more than one substance that looks different throughout. v. These are given symbols like ‘Ar’, ‘Zn’, ‘W’, and ‘Xe’ and are arranged in a table called the Periodic Table 4. Identify the following as either an ELEMENT (E), COMPOUND (C), HOMOGENEOUS MIXTURE (S) or HETEROGENEOUS MIXTURE (H) a. Vinegar and Oil Label the following on a periodic table: (check out b. Dry Ice (solid carbon dioxide (CO2)) http://www.ptable.com for additional help) c. Brass allow (Cu and Zn) d. Table Salt (NaCl) Metals e. Oxygen gas (O2) Nonmetals f. Salt dissolved in water Metalloids g. Chex Mix Transition metals h. Gold Bracelet (Au) i. Clear apply juice Inner transition metals j. Sugar (C12H22O11) Alkali metals k. Sugar dissolved in water Alkaline earth metals l. Air Halogens Noble gases (inert gases) Stair case Show which way are periods Show which way are groups/families