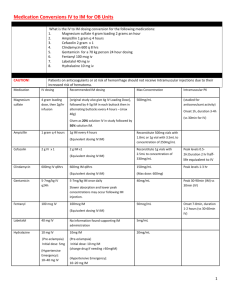
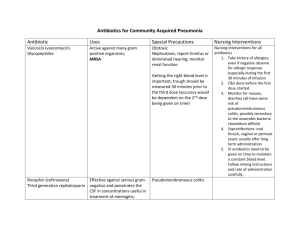
Antimicrobials
advertisement

Bug Juice 101 Deanna Moore, MSN, ACNP-BC, CCRN, NREMT-P Objectives • Review taxonomy of microorganisms • Describe lab tests used for speciation • Review antibiotic classes, mechanism of action, spectrum of activity, and dosing considerations • Review common infections encountered in the hospital setting and appropriate treatment Taxonomy - Bacterial • • • • • • Gram Stain Acid-Fast Stain Morphology Oxygen use Facultative Toxins Gram Stain • Four step process – Acid-Fast Stain • Difficult to ID with normal gram staining • Red stain does not wash off with acid alcohol – Mycobacterium • Gram Positive – Two protective layers • Phospholipid bilayer – Protects bacteria • Peptidoglycan cell wall – Allows passage of antimicrobials • Gram Negative – Three layers • LPS – protein, Lipid A – Endotoxin • More difficult to tx Lab Biomedical Tests • • • • • • • Catalase – defense against h2o2 and superoxide Citrate – utilize citrate as only carbon source Coagulase – determine if pathogenic Indole – ability to break down tryptophan Optichin – Id Streptococcus pneumo Oxidase – Presence of cytochrome oxidase Urease – enzyme that breaks C-N bond (proteus) Coagulase Test – Differientiating between pathogenic strains of Staphylococcus Catalase Oxygen Use • • • • Obligate aerobes Facultative anaerobes Microaerophilic Obligate anaerobes Gram Positive Cocci • Streptococci – catalase negative, microaerophilic – Group A beta hemolytic • “strep” throat, Skin infections – necrotizing fascitis – Group D alpha hemolytic • Enterococcus faecalis, faecium – UTI, bacteremia – Pneumoniae – pneumococcus • Pneumonia, meningitis, endocarditis • Staphylocci – catalase positive, facultative anaerobes – Coagulase Positive • S. aureus – skin flora – pna, sepsis, uti – Coagulase Negative • S. epidermidis –skin flora - prosthetics Gram Positive Rods • Endospore Forming – Bacillus – anthracis, cereus (food poisoning) • Regular, Non-endospore forming – Lactobacillus • Irregular, Non-Endospore forming – Cornyebacterium (diptheria in children) – fac anaerobes, cat + – Listeria – only gp to produce endotoxin – fac an, cat + • Pna immunosuppressed • Mycobacteria – Weakly gram positive – Tuberculosis – obligate aerobes Gram Negative • Aerobic Cocci – Neisseria – facultative anaerobes, encapsulated (resistant to host organism) • Meningitidis – meningococcus • Gonorrhea • Bacilli (rods) - enterics – Facultative anaerobes • • • • • • Ecoli Klebsiella Serratia Proteus Helicobacter pylori Enterobacter – Obligate aerobe • Pseudomonas Gram Negative • Nonenterics – Obligate aerobes • Bordetella pertussis • Legionella – Facultative anaerobes • Haemophilus influenzae • Coccobacilli – Acinetobacter - aerobic Anaerobic Features • Foul smelling discharge • Proximity to mucosal membrane • Necrotic tissue • Gas formation in tissue or discharge Anaerobes • Gram positive bacilli – Clostridium – – – – Botulinum Tetani Perfringens Difficile • Gram negative Bacilli – Spore forming rods -Bacteroides fragilis • peritonitis Fungi • Cell Membrane – Major steroid is ergosterol – object of antifungals • Cell Wall – Potent antigens for immune system • Capsule – Polysaccharide coating in some fungi – very antiphagocytic for human immune cells Fungal Infections • Candida albicans – Thrush, vaginitis, esophagitis • Histoplasma capsulatum – Lung • Cryptococcus neoformans – Lung, skin ulcers, HIV • Aspergillus flavus – Lung Brief Review - Match • • • • • • Enterococcus? Coag positive staph? Gram positive rod? Gram negative enteric? Gram negative nonenteric Gram negative coccibacillus • Gram positive rod anaerobe • • • • • • • Acinetobacter H. flu Klebsiella Streptococcus MRSA Lactobacillus C diff As said by J.B.S. Haldane….. • The danger with germkilling drugs is that they may kill the patient as well as the germ. Antimicrobials by MOA • BACTERIOSTATIC – – – – – – – Control Inhibit protein synthesis Intact immune system Tetracyclines Doxycycline Macrolides Sulfonamides • BACTERIOCIDAL – – – – – – – – – – – Kill Cell wall inhibitors PCN Vanc Cephalosporins Carbapenems Aminoglycosides Monobactams Fluoroquinolones Metronidazole Isoniazid, Rifampin Some drugs can be either based on bacteria and drug concentration Beta-Lactams/Cell Wall Inhibitors • PCN – Specific step in cell wall synthesis – Only bactericidal if cells are actively synthesizing cell wall – Resistance • Inactivation by Beta lactamase – most common – More than 300 • S.aureus, H. flu, Ecoli – still sensative to cephalosporins • Pseudomonas – both PCN and CPN • Modification of target PCN binding proteins • Impaired penetration of drug • Presence of efflux pump • PCN G – 1929 – Alexander Fleming – Streptococci, meningococci, enterococci, PCN susceptible pneumococci, non Betalactamase producing staphylococci – Lots of resistance – limited use now • PCN resistant to staphlococcal beta lactamase – Methicillin, Nafcillin • Systemic staphylococcal – – oxacillin, nafcillin 8-12 g/d (1-2 q 6h) • Methicillin - nephrotoxicity Extended Spectrum PCNs • Spectrum of PCN G + Gram Negative – Penetrate outer membrane – Inactivated by beta lactamases • Aminopenicillins – ampicillin and amoxicillin – PO/IV, UTIS, Respiratory, OM, sinusitis • Not effective against klebsiella, pseudomonas, enterobacter, citrobacter, serratia • Carboxypenicillins – carbenicillin and ticarcillin – Amp spectrum + pseudomonas, enterobacter (ticarcillin) • Ureidopenicillins – azlocillin, mezlocillin and pipericillin – Above + klebsiella • Combo drugs: + betalactamase inhibitors – clavulanic acid, sulbactam, tazobactom – increases spectrum Betalactamase prod S. aureus, Gram neg – Most common – pipercillin-tazobactam –– synergistic activity (8:1) – 3.375 g q 6h – 2.25 g q 8h (renal insufficiency) – Empiric for UTI and intraabdominal sepsis, empiric tx of neutropenic patients with fever Cephalosporins • First generation: – Aerobic GPC • Not MRSA, Staph Epi • i.e. cefazolin (Ancef) • Second generation: – Increased act against aerobic and anaerobic GNR - enteric • cefoxitin • Third generation: – Greater activity against GNR, including P. aeruginosa and H. Flu, less active against aerobic GPC than 1st generation • Ceftriaxone (Rocephin) – severe CAP • Ceftazidime (Fortez) – antipseudomonal – no serious adverse effects • Fourth generation: – Broad spectrum • Cefepime – (Pseudomonas, streptococci, MRSA) Cephalosporin Dosing Considerations • Adjust dose in renal failure – extend interval rather than deceasing amount – Preserve concentration-dependent bacterial killing – Ceftriaxone requires no dose adjustment in renal failure • Toxicity – adverse reactions uncommon and nonspecific (rash, N/D) • Risk for superinfection with 2/3 generation – MRSA, enterococcus + fungi • 5-15% cross reaction with PCN – avoid with previous anaphylactic reaction to PCN Carbepenems • Imipenem – effective against all but MRSA, C. diff, Enterococcus – Inactivated by enzymes on luminal surface of proximal renal tubules • Impossible to achieve high levels in urine • Cilastatin – enzyme inhibitor • Meropenem – broadest spectrum – Slightly superior to imipenem secondary to side effect profile but clinical experience is limited Dosing Considerations • Imipenem dosing – – – – 500 mg q 6h 1000 mg q 6h – pseudomonas Renal failure – reduced by 50-75% Generalized seizures in 1-3% • Most often in those with seizure disorder, mass, renal failure • Max daily dose 2g or 25 mg/kg • Meropenem dosing – 1 g q 8h – Dose reduction of 50% in renal failure • Ertapenem – 1 g q 24 – Standard dosing inadequate in obesity • ? Allergy if PCN allergy Other Beta Lactams • Monobactams – – – – Resistant to beta lactamases Active GNR inc pseudomonas, serratia No activity against GP or anerobes Aztreonam • Resembles aminoglycosides, q8h dosing • Tolerated by PCN allergic Vancomycin • Gram positive cocci – all strains of S. aureus (coagulase +, -, MSSA, MRSA), aerobic and anaerobic streptococci • Active against C. diff • Enterococcal resistance 1-15% Dosing Considerations • 1 g q 12 h • Infused slowly – 10mg/min • Continous infusions can achieve bactericidal drug levels in blood • Dose reduction in renal insufficiency – increase dose interval • Trough 5-15, need 20 for CNS, lung Toxicity • Red Man Syndrome – Rapid administration – vasodilation, flushing, hypotension – secondary to histamine release • Ototoxicity – Reversible hearing loss for high freq at high levels (>40) – Permanent deafness at 80 • Nephrotoxicity – 5% - no apparent relationship with dose – Higher when used with aminoglycosides – Usually returns to normal after cessation of tx Protein Synthesis Inhibitors • Bind to or interfere with ribosomes of bacteria • Tetracyclines • Macrolides • Clindamycin Tetracyclines • Broad spectrum bacteriostatic – Inhibit protein synthesis • Gram positive + gram negative + anaerobes • Resistance: – decreased IC accumulation, ribosone protection, enzymatic inactivations • Short acting (tetra), intermediate acting, Long acting (doxy, mino) • Mycoplasma pneumoniae, chlamydiae, rickettsiae, H pylori • Adverse effects – Superinfection – pseudomonas, proteus, staphylococci, clostridia, candida – Hepatotoxicity, nephrotoxicity, venous thrombosis, photosensitivity, vestibular sx, calcium chelation • Doxycycline – No renal adjustment required – Antiseizure meds, barbituates, etoh – decrease half life Macrolides • May be used for strep/staph in patients allergic to PCN, Cephalosporins • PO/IV • Erythromycin, clarithromycin, azithromycin • Few side effects – majority with e-mycin – Gut motility, hepatotoxicity – acute cholestatic hepatitis • Inhibit P450 (except azithromycin) – theophylline, warfarin, cyclosporine, methylprednisone, digoxin Clindamycin • Inhibits protein synthesis • Anaerobes from penetrating wounds of abdomen • Dental prophylaxis • Female genital tract • PO/IV • Adverse effects: GI upset, impaired liver function, neutropenia, C DIFF Antifolate, DNA Gyrase Inhibitors • Antifolate – Inhibit growth by reversibly blocking folic acid synthesis – Sulfonamides – Trimethoprim • DNA Gyrase Inhibitors – Block bacterial DNA synthesis – Fluorinated allow for better systemic levels – Fluoroquinolones Sulfonamides/Trimethoprim • Sulfamethoxazole/Trimethoprim (TMPSMX) • Synergy for wide gram +/gram – coverage • S. pneumo, H. Flu, Enteric UTIs • PO/IV • Side effects: n/v/d, hemolytic or aplastic anemia, thrombocytopenia, skin rash – can be SJS Fluoroquinolones • -1987/1996 • Two generations – differ in pharmacokinetics and spectrum – Early – Ciprofloxaxin – staphylocci (MRSA), most aerobic gram negative bacilli (pseudomonas) • Less active against streptococci – Newer – Levofloxacin, gatifloxacin, moxifloxacin • • • • Same spectrum as early except against pseudomonas Increased coverage streptococci, pneumococci, Myco, Hflu Lung and UTI Limited value in ICU secondary to limited action on pseudomonas and MRSA – used as part of multiple drug tx Dosing Guidelines • Cipro q 8h secondary to shorter half life • Newer q 24 • Dose adjustments required for all except moxifloxacin (liver metabolism) • Considerations: – Cipro interferes with theophylline and warfarin • Relatively safe – QT prolongation – Torsades • Cipro less effective in ICU secondary to resistance of GN • Newer agents not a choice for VAP except in early onset Protein Synthesis Inhibitors Gram Negative Spectrum • Aminoglycosides Aminoglycosides • Derived from cultures of Streptomyces – First - streptomycin – Eight drugs – Three clinically relevant: gentamicin (66), tobramycin (75) and amikacin (81) – Most active against gram negative bacilli including pseudomonas – Bactericidal – concentration dependent + postantibiotic effect – Usually reserved for immunocompromised or unstable gram negative sepsis Dosing Considerations • Based on body weight and renal function – Compare the ideal vs actual body weight and use the lower of the two for dosing • Small fraction in adipose tissue when considering body distribution – Once daily dosing except in endocarditis • Gentamycin: 4-7 mg/kg/d, P 5-10, T<2 (serratia) • Tobramycin: 3-5 mg/kg/d, P 5-10, T <2 (pseudomonas) • Amikacin: 15 mg/kg/d, P 20-30, T<5 – Cleared by kidneys – dosing is adjusted secondary to creatinine clearance Nephrotoxicity • Obligate nephrotoxins – Renal impairment will eventually develop if treatment continues – Oxidative injury in cells lining the proximal tubules – Early signs – cylindrical casts in the urine, proteinuria, inability to concentrate – Urine changes occur during the first week – Cr rises 5-7 days after initiation of therapy – Renal impairment enhanced by hypovolemia, age, preexisting renal impairment, hypokalemia, hypomagnesemia, concurrent tx with other “kidney” offenders (loops, cyclosporin, cisplatin, vanc) – Can progress to ARF but usually reversible Other adverse effects • Ototoxicity – Irreversible hearing loss and vestibular damage • Usually not apparent to patient • Can block Ach release from presynaptic nerve terminals – usually not apparent with therapeutic dosing – Small risk with MG and NDMA Metronidazole • Anaerobes • C. diff, Bacteroides • Side effects: No alcohol – disulfiram reaction, GI upset, neutropenia, paresthesias, caution in CNS disease • Drug interactions – Coumadin – increased INR – Antiseizure meds – increase elimination of metronidazole – Do not use in pregnancy Linezolid • Newest abx – 2000 – synthetic • Bacteriostatic except bactericidal to streptococci • Inhibits protein synthesis by prevent ribosome complex formation • Resistant gram positive organisms • Initially only recommended when vancomycin not effective or tolerated • May be replacement for vancomycin – MRSA pneumonia due to penetration into respiratory secretions Dosing Considerations • • • • 600 mg BID IV = PO Safe in short courses Longer courses (>1 month) – Thrombocytopenia – Peripheral and optic neuropathy – Peripheral is irreversible, optic partially resolves Antifungals • Amphotericin B – Naturally occuring – fungicidal for most pathogenic fungi in humans – Most effective yet most toxic • Triazoles – Synthetic antifungals – Less toxic – Fluconazole, itraconazole, voriconazole • Echinocandins – New class for invasive candidiasis – Improved spectrum, less drug interactions, no dose modifications – Capsofungin Amphotericin B - AmB • IV use only – vehicle to enhance solubility in plasma • Once daily dosing – 0.5-1mg/kg • Delivered over 4 hours (may be reduced to 1 if tolerated) • Daily infusions until cumulative dose is achieved – total dose is determined by type and severity of infection – 500 mg – 4 g Dosing Considerations • Infusion related inflammatory response – Fever, chills, nausea, vomiting, rigors – 70% – Cytokine release from activated monocytes – Most severe on initial infusion • Pretreatment with acetaminophen 10-15mg/kg po and diphenhydramine 25 mg po/iv 30 min before. Give meperidine 25 mg iv for rigors • If symptoms not relieved with pretreatment – add hydrocortisone 0.1 mg/ml to infusion – Liposomal Amphotericin B • Lipid preparations – enhance binding to fungal cells over mammalian cells – phospholipid vesicles • Less infusion related side effects, Less nephrotoxicity • Dose 5x higher • Very Costly (10x per mg + increased dose - $16 vs. $828) • Only approved for tx of documented fungal infections in pt intolerant of standard AmB or empiric coverage in neutropenic with persistent fever Adverse Effects • Phlebitis – need central access • Nephrotoxicity – Binds to cholesterol on the surface of renal epithelial cells and produces an injury in the renal tubules – resembles RTA (distal) with increased urinary excretion of K, Mg – Azotemia 30-40% with possible progression to ARF requiring dialysis – Stablilizes with continued infusions and improves with discontinuation of therapy – Cr >3 – stop infusions – Aggravated with hypovolemia, concurrent drugs • Hypokalemia, Hypomagnesemia – – Oral mg supplementation 300-600 daily – Stop with azotemia Triazoles • Candida septicemia in ICU - Fluconazole – Hemodynamically stable, Immunocompentent • Voriconazole – Aspergillosis – Emperic treatment of neutropenic with persistent fever • Itraconazole – Invasive aspergillosis – Doesn’t play well with others Dosing • Fluconazole – – – – PO = IV 400-800 daily dose, double 1st dose to decrease time to steady state (4-5d) Adjust for renal impairment, Decrease by 50% for CrCl <50 ml/min Interactions: inhibit cytochrome P450 • Phenytoin, statins, warfarin – No serious toxicity – • Elevated liver enzymes in 10% • Voriconazole – – – – – Loading 6 mg/kg q 12 for 24 h, then 4 mg/kg q 12h IV 200 mg po BID – mx dosing Long infusion – 1-2 h Well tolerated with minor GI side effects Elevated liver enzymes with combo with cyclosporin Echinocandins • New class – invasive candidiasis – Improved coverage for all Candida species – Less risk of drug interactions – No dose modifications • Caspofungin – Comparable to AmB • IV 70 mg initially, then 50 mg daily • Increased dose needed with some antivirals, rifampin, dexamethasone, antiseizure meds Duration of Therapy • Bacteremia – • After acute inflammation Cystitis – Pyelo 14 d (7 with cipro) 42 d GI – Cdiff – H pylori • 3d (single dose cipro ER) Bone – Osteomyelitis • 3d Kidney – • 10-14 d Cellulitis – • Removable device 10-14 d 10-14 d Lung – Pneumococcal – Enterobacter/pseudomonal – Staphylcoccal 5 day (afebrile 3-5) 21 – 42 d 21-28 d What about pregnancy? • • • • • • • • • Aminoglycosides Erythomycin Metronidazole Nitrofuratoin Sulfonamides/Trimethoprim Tetracycle Fluoroquinolines Vancomycin Azoles Treatments - Match • • • • • • • • Cellulitis UTI – uncomplicated UTI - complicated CAP – hospitalized CAP – ICU VAP C. diff Candida • • • • • • • • Metronidazole PO Ancef, PCN Ceftriazone + azithro Bactrim Diflucan Carbepenem + Vanc FQ Amp + gent/zosyn Questions????????? References • www.cartoonstock.com • Gilbert, D.N., Moellering, R.C., Eliopoulos, G.M., and Sande, M.A. The Sanford Guide to Antimicrobial Therapy 2007. • Hull, M. Clostridium difficile - associated colitis, 2004 • Marino, P.L. The ICU Book. 2007 • Katzung, B. Basic and Clinical Pharmacology. 2004 • Malvinder, P. Pneumocephalus associated with Bacteroides fragilis meningitis. Journal of Postgraduate medicine, 2004. • Talaro, A. and Talaro, K. Foundations in Microbiology.1996