PHall AP info. NOT integrated into this presentation
Chapter 4.6-4.10
Types of Chemical
Reactions and Solution
Stoichiometry
Chapter 4
Table of Contents
4.6
4.7
4.8
4.9
4.10
Describing Reactions in Solution
Stoichiometry of Precipitation Reactions
Acid–Base Reactions
Oxidation–Reduction Reactions
Balancing Oxidation–Reduction Equations
Copyright © Cengage Learning. All rights reserved
2
Chapter 4
Table of Contents
• Assignments - Tuesday - January 29, 2013
• CW: Notes 4.6-4.10
• CW: Pre-lab for tomorrow determining %iodine in iodine
tincture using titrations
• HW: Last week’s titration lab report due tomorrow
• HW: Ch. 4 homework problems due Thursday
• Test ch.3-4 on Friday.
3
Section 4.6
Describing Reactions in Solution
Formula Equation (Molecular Equation)
•
•
•
Gives the overall reaction stoichiometry
but not necessarily the actual forms of
the reactants and products in solution.
Reactants and products generally shown
as compounds.
Use solubility rules to determine which
compounds are aqueous and which
compounds are solids.
AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
Return to TOC
Copyright © Cengage Learning. All rights reserved
25
Section 4.6
Describing Reactions in Solution
Complete Ionic Equation
•
Represents as ions all reactants and
products that are strong electrolytes.
Ag+(aq) + NO3 (aq) + Na+(aq) + Cl (aq)
AgCl(s) + Na+(aq) + NO3 (aq)
Return to TOC
Copyright © Cengage Learning. All rights reserved
26
Section 4.6
Describing Reactions in Solution
Net Ionic Equation
•
Includes only those solution components
undergoing a change.
Show only components that actually react.
Ag+(aq) + Cl (aq) AgCl(s)
•
Spectator ions are not included (ions that
do not participate directly in the
reaction).
Na+ and NO3 are spectator ions.
Return to TOC
Copyright © Cengage Learning. All rights reserved
27
Section 4.6
Describing Reactions in Solution
Concept Check
Write the correct formula equation, complete ionic equation,
and net ionic equation for the reaction between cobalt(II)
chloride and sodium hydroxide.
Formula Equation:
CoCl2(aq) + 2NaOH(aq) Co(OH)2(s) + 2NaCl(aq)
Complete Ionic Equation:
Co2+(aq) + 2Cl (aq) + 2Na+(aq) + 2OH (aq)
Co(OH)2(s) + 2Na+(aq) + 2Cl (aq)
Net Ionic Equation:
Co2+(aq) + 2OH (aq) Co(OH)2(s)
Copyright © Cengage Learning. All rights reserved
Return to TOC
28
Section 4.7
Stoichiometry of Precipitation Reactions
Solving Stoichiometry Problems for Reactions in Solution
1. Identify the species present in the combined
solution, and determine what reaction if any
occurs.
2. Write the balanced net ionic equation for the
reaction.
3. Calculate the moles of reactants.
4. Determine which reactant is limiting.
5. Calculate the moles of product(s), as required.
6. Convert to grams or other units, as required.
Return to TOC
Copyright © Cengage Learning. All rights reserved
29
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part I)
10.0 mL of a 0.30 M sodium phosphate
solution reacts with 20.0 mL of a
0.20 M lead(II) nitrate solution (assume no
volume change).
1) What precipitate will form?
lead(II) phosphate, Pb3(PO4)2
2) What mass of precipitate will form?
Return to TOC
Copyright © Cengage Learning. All rights reserved
30
Section 4.7
Stoichiometry of Precipitation Reactions
Let’s Think About It
•
Where are we going?
•
To find the mass of solid Pb3(PO4)2 formed.
How do we get there?
What are the ions present in the combined solution?
What is the balanced net ionic equation for the
reaction?
What are the moles of reactants present in the
solution?
Which reactant is limiting?
What moles of Pb3(PO4)2 will be formed?
What mass of Pb3(PO4)2 will be formed?
Return to TOC
Copyright © Cengage Learning. All rights reserved
31
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part I)
10.0 mL of a 0.30 M sodium phosphate solution reacts
with 20.0 mL of a 0.20 M lead(II) nitrate solution
(assume no volume change).
2) What mass of precipitate will form?
2Na3PO4(aq) + 3Pb(NO3)2 (aq)
(.0100 L)(.30 mol/L)
(.0200 L) (.20 mol/L)
.0030 mol
.0040 mol
6NaNO3(aq) + Pb3(PO4)2 (s)
2:3 ratio so if you have .0030 mol you need .0045 mol, so lead II nitrate is the limiting reactant.
3:1 ratio between lead II nitrate and lead II phosphate .0040/3 = .001333 mol x 811.54 =
1.08 g = 1.1 g Pb3(PO4)2
Return to TOC
Copyright © Cengage Learning. All rights reserved
30
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part II)
10.0 mL of a 0.30 M sodium phosphate
solution reacts with 20.0 mL of a 0.20 M
lead(II) nitrate solution (assume no volume
change).
3) What is the concentration of nitrate
ions left in solution after the reaction is
complete?
Return to TOC
Copyright © Cengage Learning. All rights reserved
32
Section 4.7
Stoichiometry of Precipitation Reactions
Let’s Think About It
•
Where are we going?
•
To find the concentration of nitrate ions left in
solution after the reaction is complete.
How do we get there?
What are the moles of nitrate ions present in the
combined solution?
What is the total volume of the combined
solution?
Return to TOC
Copyright © Cengage Learning. All rights reserved
33
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part II)
10.0 mL of a 0.30 M sodium phosphate solution reacts
with 20.0 mL of a 0.20 M lead(II) nitrate solution
(assume no volume change).
3) What is the concentration of nitrate ions left in
solution after the reaction is complete?
2Na3PO4(aq) + 3Pb(NO3)2 (aq)
6NaNO3(aq) + Pb3(PO4)2 (s)
3:6 ratio reactant and product .0040 mol produces .0080 mol of nitrate ions so
.0080 mol / (.01 + .02 L) = .0080/.03 = .26666 mol/L =
0.27 M
Return to TOC
Copyright © Cengage Learning. All rights reserved
32
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part III)
10.0 mL of a 0.30 M sodium phosphate
solution reacts with 20.0 mL of a 0.20 M
lead(II) nitrate solution (assume no volume
change).
4) What is the concentration of
phosphate ions left in solution after the
reaction is complete?
Return to TOC
Copyright © Cengage Learning. All rights reserved
34
Section 4.7
Stoichiometry of Precipitation Reactions
Let’s Think About It
•
Where are we going?
•
To find the concentration of phosphate ions left in
solution after the reaction is complete.
How do we get there?
What are the moles of phosphate ions present in
the solution at the start of the reaction?
How many moles of phosphate ions were used
up in the reaction to make the solid Pb3(PO4)2?
How many moles of phosphate ions are left over
after the reaction is complete?
What is the total volume of the combined
solution?
Copyright © Cengage Learning. All rights reserved
Return to TOC
35
Section 4.7
Stoichiometry of Precipitation Reactions
Concept Check (Part III)
10.0 mL of a 0.30 M sodium phosphate solution reacts
with 20.0 mL of a 0.20 M lead(II) nitrate solution
(assume no volume change). ???? can’t get to work
out
4) What is the concentration of phosphate ions left in
solution after the reaction is complete?
2Na3PO4(aq) + 3Pb(NO3)2 (aq)
6NaNO3(aq) + Pb3(PO4)2(s)
.004 mol (ratio is 3:1) for lead II phosphate molecules but 2 phosphate ions for each molecule group, so .004/3
= moles of lead II phosphate molecules x 2 since 2 phosphate ions in each of the phosphate ions in products
(.004 mol/3)(2) = .002666 moles phosphate ions on product side
3:2 ratio on reactant side so .004 moles x 2/3 = .02666 moles phosphate ions used with reactant side but had
.003 moles available at beginning so .0030 = .02666 = .024 moles left
.024 moles/ (.01 + .02 L) =
0.011 M
Copyright © Cengage Learning. All rights reserved
Return to TOC
34
ACIDS AND BASES
www.lab-initio.com
Section 4.8
Acid–Base Reactions
Acid–Base Reactions (Brønsted–Lowry)
•
•
•
Acid—proton donor
Base—proton acceptor
For a strong acid and base reaction, the net
ionic equation is:
H+(aq) + OH–(aq) H2O(l)
Arrhenius Acid-Base Reactions
Acids - produce H+ ions in water
Bases - produce OH- ions in solution
*OH-, hydroxide ions are so strong that they can be assumed to
completely react with even a weak acid in solution
Copyright © Cengage Learning. All rights reserved
Return to TOC
36
Properties of Acids
Acids are proton (hydrogen ion, H+)
donors
Acids have a pH lower than 7
Acids taste sour
Acids effect indicators
Blue litmus turns red
Methyl orange turns red
Acids react with active metals,
producing H2
Acids react with carbonates
Acids neutralize bases
Acids are Proton (H+ ion) Donors
Strong acids are assumed to be 100%
ionized in solution (good H+ donors).
HCl
H2SO4
HNO3
Weak acids are usually less than 5%
ionized in solution (poor H+ donors).
H3PO4
HC2H3O2
Organic acids
Acids Have
a pH less
than 7
Acids
Effect
Indicators
Blue litmus paper
turns red in
contact with an
acid.
Methyl orange
turns red with
addition of an acid
Acids React with Active Metals
Acids react with active metals to form
salts and hydrogen gas.
Mg + 2HCl MgCl2 + H2(g)
Zn + 2HCl ZnCl2 + H2(g)
Mg + H2SO4 MgSO4 + H2(g)
Acids React with Carbonates
2HC2H3O2 + Na2CO3
2 NaC2H3O2 + H2O + CO2
Effects of Acid Rain on Marble
(calcium carbonate)
George Washington:
BEFORE
George Washington:
AFTER
Properties of Bases
Bases are proton (hydrogen ion, H+)
acceptors
Bases have a pH greater than 7
Bases taste bitter
Bases effect indicators
Red litmus turns blue
Phenolphthalein turns purple
Solutions of bases feel slippery
Bases neutralize acids
Bases are Proton (H+ ion) Acceptors
Sodium hydroxide (lye), NaOH
Potassium hydroxide, KOH
Magnesium hydroxide, Mg(OH)2
Calcium hydroxide (lime), Ca(OH)2
OH- (hydroxide) in base combines with H+ in
acids to form water
H+ + OH- H2O
Bases have a
pH greater
than 7
Bases Effect Indicators
Red litmus paper turns
blue in contact with a
base.
Phenolphthalein
turns bright
pink in a base.
Bases Neutralize Acids
Milk of Magnesia contains
magnesium hydroxide, Mg(OH)2,
which neutralizes stomach acid,
HCl.
2 HCl + Mg(OH)2
MgCl2 + 2 H2O
Acids Neutralize Bases
Neutralization reactions ALWAYS produce a salt
and water.
HCl + NaOH NaCl + H2O
H2SO4 + 2NaOH Na2SO4 + 2H2O
2HNO3 + Mg(OH)2 Mg(NO3)2 + 2H2O
Section 4.8
Acid–Base Reactions
Neutralization of a Strong Acid by a Strong Base
Return to TOC
Copyright © Cengage Learning. All rights reserved
37
Section 4.8
Acid–Base Reactions
Performing Calculations for Acid–Base Reactions
1. List the species present in the combined
solution before any reaction occurs, and decide
what reaction will occur.
2. Write the balanced net ionic equation for this
reaction.
3. Calculate moles of reactants.
4. Determine the limiting reactant, where
appropriate.
5. Calculate the moles of the required reactant or
product.
6. Convert to grams or volume (of solution), as
required.
Copyright © Cengage Learning. All rights reserved
Return to TOC
38
Section 4.8
Acid–Base Reactions
Acid–Base Titrations
•
•
•
Titration – delivery of a measured volume of
a solution of known concentration (the
titrant) into a solution containing the
substance being analyzed (the analyte).
Equivalence point – enough titrant added to
react exactly with the analyte.
Endpoint – the indicator changes color so
you can tell the equivalence point has been
reached.
Return to TOC
Copyright © Cengage Learning. All rights reserved
39
Section 4.8
Acid–Base Reactions
Click on the following link to watch a video and
complete an interactive titration simulation to prepare
for a future lab which is a bit different than the
previous acid-base titration.
• http://www.kentchemistry.com/links/AcidsBases/titra
tion.htm
• Complete the interactive activity and include
answers to questions in your notes.
• You may also want to watch the video on titration
above the interactive simulation.
Return to TOC
36
Section 4.8
Acid–Base Reactions
Concept Check
For the titration of sulfuric acid (H2SO4) with
sodium hydroxide (NaOH), how many moles
of sodium hydroxide would be required to
react with 1.00 L of 0.500 M sulfuric acid to
reach the endpoint?
Return to TOC
Copyright © Cengage Learning. All rights reserved
40
Section 4.8
Acid–Base Reactions
Let’s Think About It
•
Where are we going?
•
To find the moles of NaOH required for the
reaction.
How do we get there?
What are the ions present in the combined
solution? What is the reaction?
What is the balanced net ionic equation for the
reaction?
What are the moles of H+ present in the
solution?
How much OH– is required to react with all of the
H+ present?
Copyright © Cengage Learning. All rights reserved
Return to TOC
41
Section 4.8
Acid–Base Reactions
Concept Check
For the titration of sulfuric acid (H2SO4) with
sodium hydroxide (NaOH), how many moles
of sodium hydroxide would be required to
react
with without
1.00 L ofthe
0.500
<Makes
sense
net M sulfuric acid to
the endpoint?
ionicreach
equation?
H2SO4 + 2NaOH --> 2H2O + Na2SO4
2H+ + SO42- + 2Na+ + 2OH- --> 2H2O + 2Na+ SO422H+ + 2OH---> 2H2O
1.00 L x .500 M = .500 moles (1:2 ratio) with NaOH so need 1.00 moles NaOH
1.00 mol NaOH
Return to TOC
Copyright © Cengage Learning. All rights reserved
40
Section 4.8
Acid–Base Reactions
STOPPED HERE FOR TUESDAY - 1-29-13 - VOER
• STILL NEED TO GO OVER OXIDATION REDUCTION
REACTIONS.
Return to TOC
40
Chapter 4
Table of Contents
• Assignments - Tuesday - January 29, 2013
• CW: Notes 4.6-4.10
• CW: Pre-lab for tomorrow determining %iodine in iodine
tincture using titrations
• HW: Last week’s titration lab report due tomorrow
• HW: Ch. 4 homework problems due Thursday
• Test ch.3-4 on Friday.
• FINISH QUIZZES ON COMPUTER AND SHOW
SCORES
41
Chapter 4
Table of Contents
• Assignments - WEDNESDAY - January 30, 2013
• Lab: Determining %iodine in iodine tincture using
titrations
• CW: Discuss Pre-lab calculations if time permits.
• Turn in Last week’s titration lab report in box.
• HW: Ch. 4 homework problems due Thursday but will
not pick up until Friday. We have covered all but
REDOX reactions.
• Test ch.3-4 on Friday.
42
Acid-Base
Reactions
Proton Transfer
Click here to watch visualization
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Neutralization of a Strong Acid by a
Strong Base
Click here to watch visualization.
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Acid-Base Titration
Click here to watch video.
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Key Titration Terms
• Titrant - solution of known concentration
used in titration.
• Analyte - substance being analyzed.
• Equivalence point - enough titrant
added to react exactly with the analyte.
• Endpoint - the indicator changes color
so you can tell the equivalence point
has been reached.
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Performing Calculations for Acid-Base
Reactions
1. List initial species and predict
reaction.
2. Write balanced net ionic reaction.
3. Calculate moles of reactants.
4. Determine limiting reactant.
5. Calculate moles of required reactant
or product.
6. Convert to grams or volume, as
required.
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Chapter 4
Table of Contents
• Assignments - THURSDAY - January 31, 2013
• CW: REDOX reactions
• 1/2 Reactions Method of balancing REDOX rxns &
practice online activity with REDOX.
• Turned in Last week’s titration lab report in box
yesterday.
• HW: Ch. 4 homework problems turned in tomorrow
• Test ch.3-4 tomorrow - Friday - handout review
concepts
• Lab: Determining %iodine in iodine tincture using
titrations calculations for pre-lab discussed; lab report
due next Wednesday.
49
Section 4.10
Balancing Oxidation–Reduction Equations
Assignments - WED - OCT 8, 2013
• CW: Notes 4.9-4.10 (4.6 refresh - read 4.7-4.8)
• CW: REDOX Practice Problems & activities using computers
• *We will have additional time on Friday to complete this
activity.
• HW: ch. 4 p.173-175 #57, 59a, 63, 65, 67a-e, 71a-e, 73ab,
75ab, 77 due Friday.
• HW: Iodine in iodine tincture titration Lab report due Tuesday
• TEST ch.3-4 Tuesday - Oct. 15th - Test starts at 7:30 a.m.
Return to TOC
50
Oxidation-Reduction Reactions
“Redox”
LEO SAYS GER
Lose Electrons = Oxidation
Gain Electrons = Reduction
OIL RIG
Oxidation is Loss of Electrons
Reduction is Gain of Electrons
Section 4.9
Oxidation–Reduction Reactions
Redox Reactions
•
Reactions in which one or more electrons
are transferred.
Return to TOC
Copyright © Cengage Learning. All rights reserved
42
Section 4.9
Oxidation–Reduction Reactions
Reaction of Sodium and Chlorine
Return to TOC
Copyright © Cengage Learning. All rights reserved
43
Oxidation Reduction Reactions
(Redox)
•
Each sodium atom loses one electron:
•
Each chlorine atom gains one electron:
•
PLEASE NOTE THAT THE circle R should be a reaction arrow and the R should NOT
be here. This came from a different presentation and will not let me edit or delete the
R as it was made with a PPT and converted to the MAC version. I tried to add arrows
to show that this should be in it’s place by putting it on or near the R.
LEO says GER :
Lose Electrons = Oxidation
•
Sodium is oxidized
Gain Electrons = Reduction
•
OIL RIG
Oxidation is Loss of Electrons
Reduction is Gain of Electrons
Chlorine is reduced
Section 4.9
Oxidation–Reduction Reactions
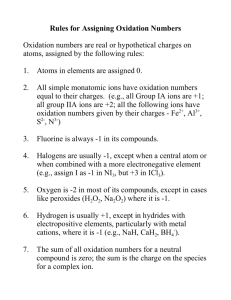
Rules for Assigning Oxidation States
1. Oxidation state of an atom in an element = 0
2. Oxidation state of monatomic ion = charge of
the ion
3. Oxygen = 2 in covalent compounds (except in
peroxides where it = 1)
4. Hydrogen = +1 in covalent compounds (-1 with
metals)
5. Fluorine = 1 in compounds
6. Sum of oxidation states = 0 in compounds
7. Sum of oxidation states = charge of the ion in
ions
Return to TOC
Copyright © Cengage Learning. All rights reserved
44
Table 4.2 Rules for Assigning Oxidation
States
Copyright © Houghton Mifflin Company. All rights reserved.
4–
Section 4.9
Oxidation–Reduction Reactions
Exercise
Find the oxidation states for each of the
elements in each of the following
compounds:
•
•
•
•
•
K2Cr2O7
CO32MnO2
PCl5
SF4
K = +1; Cr = +6; O = –2
C = +4; O = –2
Mn = +4; O = –2
P = +5; Cl = –1
S = +4; F = –1
Return to TOC
Copyright © Cengage Learning. All rights reserved
45
Examples - assigning oxidation numbers
Assign oxidation states to all elements:
59
Examples - assigning oxidation numbers
Assign oxidation states to all elements:
0
+6 -2
+1
+3 -1
+6 -2
+5 -2
-2 +1 -2 +1
+1+4 -2
+6 -2
+7
-2
+5 -2
0
60
Section 4.9
Oxidation–Reduction Reactions
Redox Characteristics
•
•
•
•
Transfer of electrons
Transfer may occur to form ions
Oxidation – increase in oxidation state
(loss of electrons); reducing agent
Reduction – decrease in oxidation state
(gain of electrons); oxidizing agent
Return to TOC
Copyright © Cengage Learning. All rights reserved
46
Section 4.9
Oxidation–Reduction Reactions
Concept Check
Which of the following are oxidation-reduction
reactions? Identify the oxidizing agent and the
reducing agent.
a)Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g)
b)Cr2O72-(aq) + 2OH-(aq) 2CrO42-(aq) + H2O(l)
c)2CuCl(aq) CuCl2(aq) + Cu(s)
Return to TOC
Copyright © Cengage Learning. All rights reserved
47
Section 4.10
Balancing Oxidation–Reduction Equations
Balancing Oxidation–Reduction Reactions by Oxidation States
1. Write the unbalanced equation.
2. Determine the oxidation states of all atoms in
the reactants and products.
3. Show electrons gained and lost using “tie
lines.”
4. Use coefficients to equalize the electrons
gained and lost.
5. Balance the rest of the equation by
inspection.
6. Add appropriate states.
Copyright © Cengage Learning. All rights reserved
Return to TOC
48
Section 4.10
Balancing Oxidation–Reduction Equations
•
Balance the reaction between solid zinc
and aqueous hydrochloric acid to
produce aqueous zinc(II) chloride and
hydrogen gas.
Return to TOC
Copyright © Cengage Learning. All rights reserved
49
Section 4.10
Balancing Oxidation–Reduction Equations
1. What is the unbalanced equation?
•
Zn(s) + HCl(aq) Zn2+(aq) + Cl–(aq) + H2(g)
Return to TOC
Copyright © Cengage Learning. All rights reserved
50
Section 4.10
Balancing Oxidation–Reduction Equations
2. What are the oxidation states for each atom?
•
Zn(s) + HCl(aq) Zn2+(aq) + Cl–(aq) + H2(g)
0
+1 –1
+2
–1
0
Return to TOC
Copyright © Cengage Learning. All rights reserved
51
Section 4.10
Balancing Oxidation–Reduction Equations
3. How are electrons gained and lost?
1 e– gained (each atom)
•
Zn(s) + HCl(aq) Zn2+(aq) + Cl–(aq) + H2(g)
0
+1 –1
+2
–1
0
2 e– lost
•
The oxidation state of chlorine remains unchanged.
Return to TOC
Copyright © Cengage Learning. All rights reserved
52
Section 4.10
Balancing Oxidation–Reduction Equations
4. What coefficients are needed to equalize the
electrons gained and lost?
1 e– gained (each atom) × 2
•
Zn(s) + HCl(aq) Zn2+(aq) + Cl–(aq) + H2(g)
0
+1 –1
+2
–1
0
2 e– lost
•
Zn(s) + 2HCl(aq) Zn2+(aq) + Cl–(aq) + H2(g)
Return to TOC
Copyright © Cengage Learning. All rights reserved
53
Section 4.10
Balancing Oxidation–Reduction Equations
5. What coefficients are needed to balance the
remaining elements?
•
Zn(s) + 2HCl(aq) Zn2+(aq) + 2Cl–(aq) + H2(g)
Return to TOC
Copyright © Cengage Learning. All rights reserved
54
20.4 Oxidation # Changes
an increase in oxidation number of an atom
signifies oxidation
+2 to +4
a decrease in oxidation number of an atom
signifies reduction
0 to -1
70
Identifying Redox Reactions
Oxidation and reduction always occur
together in a chemical reaction. For this
reason, these reactions are called “redox”
reactions.
Although there are different ways of
identifying a redox reaction, the best is to
look for a change in oxidation state:
71
I. Balancing Redox Reactions
by half-reaction method
STEP 1. Split Reaction into 2 Half-Reactions
STEP 2. Balance Elements Other than H & O
STEP 3. Balance O by Inserting H2O into eqns. as
necessary
STEP 4. Balance H with H+ or H2O (see 4a, 4b)
STEP 5. Balance Charge by Inserting Electrons
as needed
STEP 6. Multiply Each 1/2 Reaction by Factor
needed to make no. of Electrons in each 1/2
Reaction Equal
STEP 7. Add Eqns. & Cancel Out Duplicate terms,
where possible
I. Balancing Redox Reactions
(continued)
STEP 4a. In ACID: Balance H by Inserting H+, as
needed
STEP 4b. In BASE: Balance H by (i) inserting 1
H2O for each missing H & (ii) inserting same no.
of OH- on OTHER SIDE OF REACTION as
H2O’s added in (i)
Section 4.10
NEED TO UPDATE THIS!
Balancing Oxidation–Reduction Equations
Online Activity Problems - see handout sheet
• Balancing REDOX in acidic soln.- Problem #4 example do this on board using rules.
• Balancing REDOX in basic soln - Problem #5 exampledo this on board using rules.
• Students practice the online activities with these.
Return to TOC
74
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
+6 -2redox equation in acidic
-1 solution
+1 -2
Balance the following
0
+1
-2
+3
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #1 - Write the half-reactions if Redox reaction.
+6
Reduction half-rxn
Oxidation half-rxn.
+3
Cr2O72- → Cr3+
-1
0
C2H4O → C2H4O2
Return to TOC
75
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #2 - Balance the elements that are NOT oxygen &
hydrogen
Cr2O72- → 2Cr3+
C2H4O → C2H4O2
Return to TOC
76
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #3 - Balance oxygen with water
Cr2O72- → 2Cr3+ + 7H2O
H2O + C2H4O → C2H4O2
Return to TOC
77
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #4 - Balance hydrogen with hydrogen ions, H+
14H+ + Cr2O72- → 2Cr3+ + 7H2O
H2O + C2H4O → C2H4O2 + 2H+
Return to TOC
78
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #5 - Balance charges with electrons, e-
6e- + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
H2O + C2H4O → C2H4O2 + 2H+ + 2e-
Return to TOC
79
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #6 - Balance electrons lost and gained
6e- + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
(3)(H O + C H O → C H O + 2H+ + 2e-)
2
2 4
2 4 2
3H2O + 3C2H4O → 3C2H4O2 + 6H+ + 6eReturn to TOC
80
Section 4.10
Balancing Oxidation–Reduction Equations
Practice Problem #4 on handout sheet in acidic sol’n
•
Problem #4 - Redox in Acidic Solution, Overall Result
Balance the following redox equation in acidic solution
Cr2O72- + C2H4O → C2H4O2 + Cr3+
Step #7 - Sum the two half-reactions
6e- + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
3H2O + 3C2H4O → 3C2H4O2 + 6H+ + 6e8H+ + Cr2O72- 3C2H4O → 3C2H4O2 + 2Cr3+ + 4H2O
Return to TOC
81
Section 4.10
Balancing Oxidation–Reduction Equations
Online Activity Problems
• Other information, videos, and practice problems
• http://www.kentchemistry.com/aplinks/chapters/4chemrx
ns/BalancingRedox.htm
Return to TOC
82
Section 4.10
Balancing Oxidation–Reduction Equations
Assignments - WED - OCT 8, 2013
• CW: REDOX Practice Problems & activities using computers
• *We will have additional time on Friday to complete this
activity.
• HW: Be sure all notes for ch. 4 are caught up and complete.
• HW: ch. 4 p.173-175 #57, 59a, 63, 65, 67a-e, 71a-e, 73ab,
75ab, 77 due Friday.
• HW: Iodine in iodine tincture titration Lab report due Tuesday
• TEST ch.3-4 Tuesday - Oct. 15th - Test starts at 7:30 a.m.
Return to TOC
83
Chapter 4
Table of Contents
• Assignments - Tuesday - January 29, 2013
• CW: Notes 4.6-4.10
• CW: Pre-lab for tomorrow determining %iodine in iodine
tincture using titrations
• HW: Last week’s titration lab report due tomorrow
• HW: Ch. 4 homework problems due Thursday
• Test ch.3-4 on Friday.
84
Oxidation and Reduction (Redox)
Electrons are transferred
Spontaneous redox rxns can transfer
energy
Electrons (electricity)
Heat
Non-spontaneous redox rxns can be
made to happen with electricity
Not All Reactions are Redox Reactions
Reactions in which there has been no change
in oxidation number are not redox rxns.
Examples:
•
•
Rules for Assigning Oxidation Numbers
Rules 1 & 2
1. The oxidation number of any uncombined
element is zero
2. The oxidation number of a monatomic ion
equals its charge
•
Rules for Assigning Oxidation Numbers
Rules 3 & 4
3. The oxidation number of oxygen in
compounds is -2
4. The oxidation number of hydrogen in
compounds is +1
Rules for Assigning Oxidation Number
Rule 5
5. The sum of the oxidation numbers
in the formula of a compound is 0
2(+1) + (-2) = 0
H
O
(+2) + 2(-2) + 2(+1) = 0
Ca
O
H
Rules for Assigning Oxidation Numbers
Rule 6
6. The sum of the oxidation numbers in the
formula of a polyatomic ion is equal to
its charge
X + 3(-2) = -1
N
O
X = +5
X + 4(-2) = -2
S
O
X = +6
The Oxidation Number Rules - SIMPLIFIED
1. The sum of the oxidation numbers in
ANYTHING is equal to its charge
2. Hydrogen in molecular compounds is +1
3. Oxygen in compounds is -2 except with
perioxides
Reducing Agents and Oxidizing Agents
The substance reduced is the oxidizing agent
The substance oxidized is the reducing agent
•
Sodium is oxidized – it is the reducing agent
•
Chlorine is reduced – it is the oxidizing agent
Trends in Oxidation and Reduction
Active metals:
• Lose electrons easily
• Are easily oxidized
• Are strong reducing agents
Active nonmetals:
• Gain electrons easily
• Are easily reduced
• Are strong oxidizing agents
Redox Reaction Prediction #1
Important Oxidizers
Formed in reaction
MnO4- (acid solution)
MnO4- (basic solution)
MnO2 (acid solution)
Cr2O72- (acid)
CrO42HNO3, concentrated
HNO3, dilute
H2SO4, hot conc
Metallic Ions
Free Halogens
HClO4
Na2O2
H2O2
Mn(II)
MnO2
Mn(II)
Cr(III)
Cr(III)
NO2
NO
SO2
Metallous Ions
Halide ions
ClOHO2
Redox Reaction Prediction #2
Important Reducers
Formed in reaction
Halide Ions
Free Metals
Metalous Ions
Nitrite Ions
Sulfite Ions
Free Halogens (dil, basic sol)
Free Halogens (conc, basic sol)
C2O42-
Halogens
Metal Ions
Metallic ions
Nitrate Ions
SO42Hypohalite ions
Halate ions
CO2
Oxidation Reduction
Table 12.2
Strongest Oxidizing Agent
Weakest Reducing Agent
Inquiry into Chemistry
Ba 2+ (aq)
Ba (s)
Ca 2+ (aq)
Ca (s)
Mg 2+ (aq)
Mg (s)
Al 3+ (aq)
Al (s)
Zn 2+ (aq)
Zn (s)
Cr 3+ (aq)
Cr (s)
Fe 2+ (aq)
Fe (s)
Cd 2+ (aq)
Cd (s)
Tl + (aq)
Tl (s)
Co 2+ (aq)
Co (s)
Ni 2+ (aq)
Ni (s)
Sn 2+ (aq)
Sn (s)
Cu 2+ (aq)
Cu (s)
Hg 2+ (aq)
Hg (s)
Ag 2+ (aq)
Ag (s)
Pt 2+ (aq)
Pt (s)
Au 1+ (aq)
Au (s)
Weakest Oxidizing Agent
Strongest Reducing Agent
96
Chapter 4
Table of Contents
• Assignments - FRIDAY - February 1, 2013
• CW: Finish REDOX Computer Activity Problems with
• 1/2 Reactions Method of balancing REDOX rxns &
practice online activity with REDOX.
• Answer Questions on Ch. 4 homework problems may
turn in or keep until Monday to use for studying.
• CW: Computer Link Activities and Problems *-required
• Test ch.3-4- MONDAY - handout review concepts
• Lab: Determining %iodine in iodine tincture using
titrations calculations for pre-lab discussed; lab report
due next Wednesday.
• Discuss Pre-lab problems.
97
END OF
OxidationReduction
Reactions
AND Ch.4