Global Change
advertisement

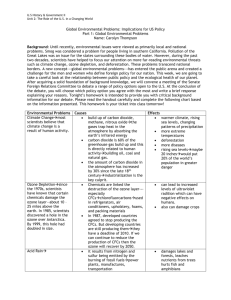
Global Change 1. Greenhouse Effect • Causes: green house gases (water vapor, carbon dioxide, methane, nitrous oxide (acid rain0, halogenated fluorocarbons, perflorinated carbons, hyddrofluorocarbons, ozone (troposphere). • Where do green house gases come from?: transportation, landfills, burning fossil fuels, digestive gases of sheep and cattle, rice, released with natural gas, • Results: Global warming Stratospheric Ozone Depletion • Causes: CFC’s • Effects: holes in good ozone which protects us from UV light. Global Climate Change Evidence • Sea levels rising • Global temps rising • Warming oceans • Shrinking ice sheets • Extreme weather events • Ocean acification • Declining artic sea • Glacial retreat Clean Air Act • In October 1948, Donora, Pennsylvania. The cloud which lingered for five days, killed 20 people and caused sickness in 6,000 of the town's 14,000 people. • In 1952, over 3,000 people died in what became known as London's "Killer Fog." The smog was so thick that buses could not run without guides walking ahead of them carrying lanterns. • Events like these alerted us to the dangers that air pollution poses to public health. Several federal and state laws were passed, including the original Clean Air Act of 1963, which established funding for the study and the cleanup of air pollution. • But there was no comprehensive federal response to address air pollution until Congress passed a much stronger Clean Air Act in 1970. That same year Congress created the EPA and gave it the primary role in carrying out the law. • In 1990, Congress dramatically revised and expanded the Clean Air Act, providing EPA even broader authority to implement and enforce regulations reducing air pollutant emissions. The 1990 Amendments also placed an increased emphasis on more costeffective approaches to reduce air pollution. 1. What is Air Pollution? • The introduction of chemicals, particulate matter or microorganisms into the air in high enough concentrations to harm plants, animals and alter ecosystems. • AKA: pollution in the troposphere. 2. Sources • Anthropogenic vs natural 3. Examples • • • • • • • • • Sulfur dioxide* Nitrogen oxide* Carbon monoxide* Carbon dioxide Particulate matter* Lead* Ozone* VOC’s Volatile organic compounds Mercury 4. Primary vs. Secondary Pollutants Acid Rain aka Acid Deposition • http://channel.nationalgeographic.com/chann el/videos/acid-rain-invisible-menace/ • http://www.youtube.com/watch?v=Nc6j7zz1_ do Pages 122-124 and 129-134 • • • • • • • • • Sulfur dioxide* Nitrogen oxide* Carbon monoxide* Carbon dioxide Particulate matter* Lead* Ozone* VOC’s Volatile organic compounds Mercury Pollutant Mercury Source (anthropogenic and natural) Coal, oil, and gold mining Effects/Impacts Impairs nervous system and bio-accumulates in the food chain. Symbol Hg Pollutant Source (anthropogenic and natural) Effects/Impacts Symbol Mercury Coal, oil, and gold mining Impairs nervous system Hg and bio-accumulates in the food chain. Sulfur dioxide Burning coal, volcanic eruptions and forest fires Respiratory irritation, harms plant tissues, converts to sulfuric acid SO2 Nitrogen oxide Cars, coal burning, lightning, fertilizer and animal waste Converts to nitric acid, component of acid rain. NO Carbon Monoxide Cars, forest fires, tobacco smoke and inefficient stoves Reduces blood’s ability to transport oxygen CO Carbon Dioxide Burning fossil fuels and deforestation Global warming, climate change CO2 Particulate matter Dust, wildfires, sea salt, coal burning, cars, plows, smoke Asthma, shorten life, reduce visibility, corrode metals PM Lead Paint, leaded gasoline Harm nervous system, palsy and MR Pb Ozone Secondary pollutant from sunlight, water, Oxygen and Major component of photochemical smog, O3 Ozone Secondary pollutant from sunlight, water, Oxygen and VOC’s Major component of photochemical smog, damages plants, heart disease and breathing problems. O3 Volatile Organic Compounds Hydrocarbons from plants, landfills, oil and natural gas wells, Leukemia, blood disorders, and immune system damage VOC’s Secondary Pollutants • Smog: • http://www.youtube.com/watch?v=f6LHtQRgdII – Industrial – Photochemical Photochemical Nitrogen based Brown-air Industrial Sulfur based Grey-air Catalyzed by UV Not catalyzed by UV Developed countries Non developed countries • Temperature Inversions and Heat Islands What can we do? • Emphasizing tax incentives for pollution control rather than fines and penalties. • Setting legislative standards for energy efficiency. • Increasing funds for research. • Distribute solar cook stoves to developing countries to replace coal and wood burning. • Phase out two-cycle gas engines. • For sick building: control materials in construction, replace and make repairs that have had water damage. Use paints, adhesives, etc in well ventilated areas, increase air circulation • Provide incentives for mass transit. Stratospheric Ozone Depletion • http://www.youtube.com/watch?v=1abN9E-Ojrc • Either a short comic strip (6 frames) or a short children’s book, or an informative brochure. • Pages 90-96 • Must include – – – – – Where is the ozone layer? Major function How are we depleting it? What effects does this have on us? What can we do about it? 5 Questions to know about stratospheric ozone depletion. 1. How is ozone formed in the atmosphere? 2. Why do we care about atmospheric ozone? 3. What is the total ozone depletion distributed over the globe? 4. What emissions from human activities lead to ozone depletion? 5. Are there controls on the production of ozone-depleting substances?