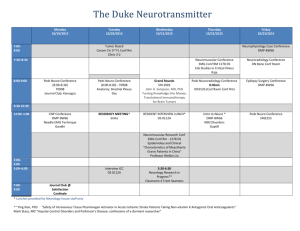
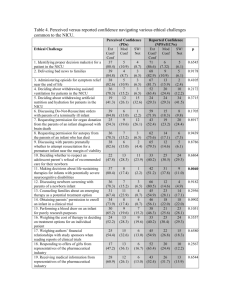
Ab Initio Quantum Chemistry: Thermochemistry and Kinetics
advertisement
New Approaches for Teaching the Chemical Principles of Engineering Phil Westmoreland University of Massachusetts Amherst westm@ecs.umass.edu “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. What are the chemical principles of engineering? • Principles that underlie the useful properties of atoms, molecules, macromolecules, continuum ensembles, materials • Is it chemistry? Is it physics? Is it biology? • Biochemistry? Physical chemistry? Chemical physics? • Physical organic chemistry? Biophysical chemistry? • Quantum mechanics? Statistical mechanics? • Semiconductor physics? Organic semiconductors? • I don’t care! These are all “molecular sciences”. • Student need to master these principles - and can. • Molecular modeling and computer visualization helps greatly! “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. ChE is the engineering profession that focuses on applying chemistry. • Phase and reaction equilibria – Bond and interaction energies – Ideal-gas thermochemistry – Thermochemistry and equations of state for real gases, liquids, solids, mixtures – Adsorption and solvation • Reaction kinetics – Rate constants, products – Metabolic pathways • Transport properties – Interaction energies, dipole – µ, kthermal, DAB • Analytical information – Spectroscopy: Frequencies, UV / Vis /IR absorptivity – GC elution times – Mass spectrometric ionization potentials and cross-sections, fragmentation patterns – NMR shifts • Protein folding and misfolding, docking, ADME, drug discovery • Mechanical properties of hard and soft condensed matter • Electronic & optical properties “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Yes, ChE is the engineering profession that focuses on applying chemistry. • But mechanical engineers use many properties that are molecular in origin. – Basic thermochemistry: ∆fHº, Cpº, Sº – P-V-T relations – Strength of materials • So do civil and environmental engineers – Chemical and biological treatment – Air, water, soil properties – Effects of environment on materials • And likewise electrical and computer engineers – Band gap as collective HOMO-LUMO differences. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. We all need to understand the chemical principles of engineering because we all need properties. • Maybe accurate, precisely known numbers. – Necessary for accurate design, costing, safety analysis. – Cost and time for calculation may be secondary. • Maybe “just” accurate trends and estimates. – – – – Often more valuable. Correlate with data to get high-accuracy predictions. Use to identify relationships between structure and properties. Enormous value for product and process development, operations, and troubleshooting. • Great data are best, but we must understand enough theory to predicting unmeasured properties. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. 1. Most property predictions are by correlations, wholly empirical or theory-based. • Arrhenius kinetics • Ideal gas law • Ideal gas mixtures (P=xiP= Pi): • Ideal solutions • Activity coefficients Ken Jolls, www.public.iastate.edu/~jolls/gibbsPics/pvtn.jpg “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. 2. Property correlations require a grasp of underlying principles. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. 3. Molecular visualization helps develop this grasp. Compare the descriptions: i-Pr H3 C N (C33N3H43)FeCl2, a liganded di(methyl imide xylenyl) aniline ... Fe N i-Pr Cl Cl N i-Pr H3 C i-Pr “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. See functionality with the 3-D structure. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. A key tool for describing molecular biology… “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Such as enzymatic docking. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. 5. For getting and using quantitative correlations properly, use the appropriate theory. Continuum Mechanics 1m 100 m 0.1 m 10 nm Statistical Mechanics Length 1 nm Quantum Mechanics 10 ns 1 ps Time 1 hr (After Maroudas, 2002) Molecular and material structure Electronic structure theory (Computational quantum chemistry) "Ab initio": Wavefunction methods Density-functional theory Molecular simulations Monte Carlo simulations Quantum MD, Car-Parrinello Molecular dynamics, molecular mechanics Semi-empirical MO theory Basis sets Statistical mechanics Potential energy functions Thermochemistry, kinetics, transport, materials properties, VLE, solutions 6. We can use these computational tools to help us teach about theory and applications. The educational principle: • Easy visualization and successful predictions motivate students to study useful underlying theories. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Example: Sketch ethylene; Calculate optimized structure/frequencies/thermo; Compare to data. Electron density HOMO; LUMO Calculations and graphics at HF/3-21G* with MacSpartan Plus (Wavefunction Inc.). “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Then they’ll tackle “How” -- the needed theory. • Maxwell-Boltzmann and Bose-Einstein statistics. • Ideal-gas thermochemistry for Cpº and Sº, broken down into additive translation, rotation, vibration: q(V,T ) ln q(V,T ) S N 1 ln T N V T ln qtransqrotqvibrqelec S(molar ) R ln qtransqrotqvibr qelec e T V T Strans Srot Svibr Selec • Compare with group additivity correlations. • [Can develop transition-state theory quickly, logically.] “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Get bond lengths, bond angles, frequencies from analogies -- or from quantum chemistry. • Efficiently explain the underlying quantum chemistry. • Easiest to think of a small, covalently bonded molecule like H2 or CH4 in vacuo. With energies, • Most simply, the goal of electronic structure w e can optimize calculations is energy. molecular structure Energy E [from • However, usually we want x EH ] energy of an optimized x structure and the energy’s variation with structure. x Position r “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. For quantum mechanics, a Hamiltonian operator is used for translational + kinetic energy. • Obtain a Hamiltonian function for a wave using the Hamiltonian operator: h2 2 H U(x, y, z) 8m to obtain: ih (q ,t) E H (q,t) 2 t where is the “wavefunction,” an eigenfunction of the equation • Born recognized that 2 is the probability density function “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. H-atom eigenfunctions y correspond to hydrogenic atomic orbitals. l=0 m=0 z l=1 m=-1 m=0 l=2 m=+1 n=1 1s n=2 2s 2px 2py 2pz n=3 3s 3px 3py 3pz m=-2,-1,0,1,2 3d orbitals (5) y x “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Construct each MO yi by LCAO. • Lennard-Jones (1929) proposed treating molecular orbitals as linear combinations of atomic orbitals (LCAO): y i Ci i i 1 • Linear combination of s orbital on one atom with s or p orbital on another gives s bond: • Linear combination of p orbital on one atom with p orbital on another gives bond: “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Simulate the real functionality with gaussians. • Start with a function that describes hydrogenic orbitals well. 3 – Slater functions are “best”; e.g., – Gaussian functions are better; e.g., • No s cusp at r=0 • However, all analytical integrals – Linear combinations of gaussians; e.g., STO-3G • 3 Gaussian “primitives” to simulate a STO • (“Minimal basis set”) 1s (r ; 1 ) 1 exp(1 r ) 3/ 4 2 2 gs r ; exp( r ) True STO 3 gaussian functions r “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Then explain Hartree-Fock, density functional theory, compound methods, and then... Improved electron correlation H-F MP2 MP4 QCISD(T) ... Full CI STO-3G 3-21G More complete 6-31G(d) basis 6-311G(d,p) sets 6-311+G(d,p) Infinite basis set Exact solution “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. 7. Use them to solve some small, real problems that reinforce the point. • Heat of combustion for dimethyloxirane safety. • Rate constant for simple reaction like C2H4+OH. • Heat of solvation for small molecules in various solvents. • Fit Lennard-Jones parameters for simple potential. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Safety / reactor engineering example: “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. Simplest properties are interaction energies: Here, the van der Waals well for an Ar dimer. 100.00 Basis set: aug-cc-pVDZ; Method: HF 50.00 MP2 0.00 MP3 0 2 4 6 8 10 MP4D MP4DQ -50.00 MP4SDQ CCSD -100.00 -0.2 kcal/mol = -0.8 kJ/mol CCSD(T) L-J 12-6 -150.00 Ar-Ar, angstroms “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. In conclusion, We can use these tools effectively to teach the chemical principles our students will need. • Build on students’ chemistry education and their prior use of properties to solve problems. • Refresh their recognitions of molecule types using sketching / visualization codes. • Have them predict structures and properties. • With them motivated, build the underlying theory. • Have them obtain properties for use. "Chemistry and life sciences in a new vision of chemical engineering," Chemical Engineering Education, 35(4), 248-255 (2001). http://www.et.byu.edu/~rowley/WebModules/modules.htm “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006. “New Approaches for Teaching the Chemical Principles of Engineering.” New England Section Conf., March 17-18, 2006.