Lab-Electrochemical Cell

Name
Electrochemical Cell
Objective
To build and operate an electrochemical cell
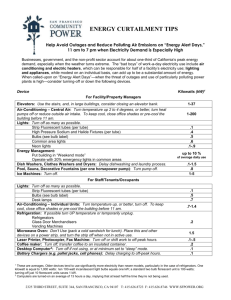
Materials
Date
CuSO4 (Copper Sulfate) NaSO4 (Sodium Sulfate) Beaker
Copper Strip Magnesium Strip Dialysis Tubing Wiring
Bulb
Procedure
1.
Put 100 mL of NaSO4 in beaker
2.
Put 50 mL of CuSO4 in dialysis tubing
3.
Gently place dialysis tubing in beaker but do NOT mix solutions
4.
Attach wires to bulb and metal strips.
5.
Place copper strip in CuSO4
6.
Place magnesium strip in NaSO4
7.
Notice if bulb lights up. If you do NOT see the bulb light up ask Mrs. S for the ammeter to monitor voltage.
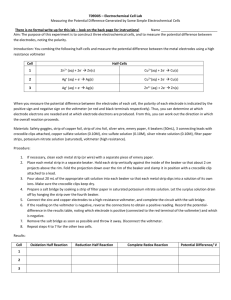
Results
Did bulb light up? Yes/No
Conclusion
Questions
1.
Which metal was the anode?
2.
Which metal was the cathode?
3.
Write the half reaction for what happened at the anode.
4.
Write the half reaction for the cathode
5.
What is a redox reaction?
6.
What is an electrochemical cell?
7. Determine if the following are redox reactions. If they are state what is getting oxidized and what is getting reduced.