Olfaction
advertisement

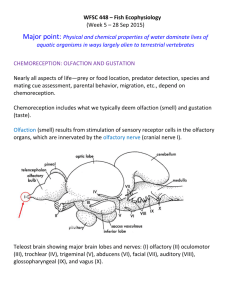
Chemical Senses: Olfaction and Taste • Dedicated to detection of chemicals in our environment. • Differ from other modalities in that the stimuli they detect cannot be easily classified by a quantitative scale. Olfaction • Monitors chemical environment at a distance, unlike taste. • Humans distinguish 1,000’s of different odors. • Mediates food selection, avoidance of ingesting toxins • Important in social cues including reproductive behavior and mother—infant relationship Vomeronasal organ is rudimentary in humans Olfactory bulb and tract Vomeronasal nerve and organ Vomeronasal organ detects pheromones in many animals where it projects to accessory olfactory bulb and then to hypothalamus. It is apparently not functional in humans and is present bilaterally in only 8% of adults. Pheromones? Nolte, p. 332 Olfactory epithelium Olfactory epithelium contains olfactory receptor neurons and supporting cells. Olfactory epithelium 1-2 cm2 Olfactory bulb & tract Bowman’s glands secrete mucus that covers surface of sensory epithelium Nolte, p. 330 Human olfactory epithelium showing chemosensory cilia • • • Only visual system has more receptor cells Total surface area of cilia is estimated to be: – 22 cm2 in human – 7 m2 in German shepard! Olfactory neurons replaced every 60 days Short microvilli on supporting cells Nolte p. 333 Cilia on olfactory receptor Olfactory receptors Cilia Dendrite Axon There are about 950 odorant receptor genes in humans, but about 60% are not transcribed, leaving ~400 functional odorant receptors. There are odorant receptor genes on each human chromosome except chromosomes 20, 22 and the Y chromosome. How olfactory information is coded is still poorly understood. In mammals each receptor neuron probably only expresses a single receptor type, but some receptors respond to more than one odorant molecule. Ligand specificity is known for only 1 mammalian odorant receptor. It is the aldehyde n-octanal, which smells like freshly cut grass. Ligand depolarizes receptor, causing action potentials travel along receptor axon to glomerulus in olfactory bulb Nolte, p. 334 Olfactory transduction Odorant binding to receptor activates G protein > activates adenylate cyclase > increases intracellular [cAMP] > opens cyclic nucleotide gated channels > Na+ & Ca2+ enter > Ca2+ opens Cl- channel that further depolarizes receptor Odorants bind to receptors with 7 transmembrane domains characteristic of Gprotein receptors. Domains 3 – 5 are highly variable and probably odorant binding sites. Binding may be direct or via odorant binding proteins in mucus that sequester odorant and may shuttle it to receptors http://www.cf.ac.uk/biosi/staff/jacob/teaching/sensory/olfact1.html Recognition of chemicals by olfactory system • • • • • • Nose can distinguish very similar compounds as different smells For example, the two stereoisomers of carvone smell like spearmint and caraway This implies there are stereoisomer-specific carvone receptors Schemes classifying odorants in categories such as ‘pungent, floral, musky’ etc. are empirical and not based on known receptor coding. Bell pepper odorant can be detected at concentration of 0.01 nM Threshold is lower for lipid soluble odorants than for water soluble odorants. Carvone CH3 O CH3 CH2 Each glomerulus gets axons from one receptor type Olfactory tract Mitral cells are principal projection neurons of the olfactory bulb Mitral cell Glomerulus Receptor cell Nolte, p. 336 Olfactory epithelium Central olfactory pathways Lateral olfactory tract projects directly to the piriform cortex (= primary olfactory cortex = paleocortex) adjacent to lateral olfactory tract in temporal lobe. This is only sense that does not have relay in thalamus on way from receptors to cerebral cortex. From piriform cortex there are projections to hypothalamus, the thalamus, amygdala, entorhinal cortex. From thalamus there is a projection to orbitofrontal cortex where odor perception and discrimination takes place. Electrical stimulation of piriform cortex causes olfactory sensations. People with lesions of orbitofrontal cortex are unable to discriminate odors. Pathways through amygdala and hypothalamus mediate emotional, motivational and many physiological effects of odors. Fix, p. 326 Olfactory hallucinations and uncinate seizures • Olfactory hallucinations can be the result of temporal lobe seizures; they are often part of the “aura” that precedes a seizure. May be accompanied by chewing or lip smacking. Olfactory memories • Odors are potent contextual cues for memory formation, emotional conditioning, olfactory flashbacks. Trauma-related smells precipitate flashbacks in patients with post-traumatic stress disorder. • Identification of odors likely involves matching them to memory templates stored in brain. A smell is categorized based on one’s previous experiences of it and on the other sensory stimuli that correlate with its appearance. Anosmia Temporary or permanent loss of sense of smell • Conductive: nasal polyps, septal deviation, inflammation • Sensorineural: head trauma, toxic chemicals, allergic reactions, neuro- degenerative diseases such as Alzheimer’s or Parkinson’s • Tumor beneath orbital surface of frontal lobe can produce unilateral anosmia • Age: Reduced sensitivity due to altered vascularity, mucus composition, changes in innervation. Taste Taste provides information about quality, quantity and safety of ingested food. Papillae distributed over tongue. Types: Fungiform: anterior tongue; ~200. Facial nerve. Foliate: lateral edge; mainly sour; glossopharyngeal n. Circumvallate: sour, bitter; back of tongue Receptor cells in taste buds on papillae and also epiglottis, soft palate, pharynx Circumvallate papilla Taste buds Nolte, p 324-325 Papillae Taste Buds, Receptor Cells • • • Taste buds have 3 cells types: receptor, supporting, and basal cells Receptor cells do not have axons, innervated by nerve fibers Receptor cells live only about 2 weeks. Basal cells differentiate into new receptor cells. There are five basic tastes with different transduction mechanisms • • • • Salt: Na ions enter receptor via sodium channels, depolarize, transmitter release Sour: H+ blocks potassium channels Sweet and Bitter: involve G proteins & second messengers Umami: Amino acids, e.g., glutamate, aspartate; MSG. Metabotropic glutamate G protein receptors and ionotropic glutamate receptors. Artificial sweeteners All taste sweet but structures differ Sugars and artificial sweeteners activate different second messenger systems in same receptor cell. Sugars activate cyclic nucleotide cascade leading to increase in cAMP. Artificial sweeteners activate IP3 (inositol-1, 4, 5-trisphosphate) system. Supertasters % of population Density of taste papillae at tip of tongue cm-2 ________________________________________________________________________________________ Supertasters 25 165 Normal tasters 50 127 Non-tasters 25 117 __________________________________________________________ Bitter compound: phenylthiocarbamide (PTC) contains N—C=S group. Single autosomal gene. Innervation of taste buds • Taste receptors in tongue mucosa, few on epiglottis and pharynx • Ganglion neurons in cranial nerve ganglia of VII, IX, and X • Relay neurons in medulla Blue: VII.Facial n. geniculate ganglion Nucleus of solitary tract Green: IX. glossopharyngeal n. inferior ganglion Red: X. Vagus n. inferior ganglion Nolte p 327 Taste pathways in CNS Nucleus of the solitary tract projects to VPM of thalamus and to parabrachial nucleus in the pons. Parabrachial nucleus projects to hypothalamus (H) [and amygdala (A) in most mammals, but maybe not in primates.] DMN X = dorsal motor nucleus of vagus. Nolte p. 329 How are different tastes coded in the brain? The “Across Fiber Pattern theory” • There are many more taste qualities than the traditional ‘primary’ tastes • Taste quality is coded by a pattern of simultaneous activity of a number of nerve fibers. • There are many types of nerve fibers • The same nerve fiber may respond to several different tastants