Chemistry Groupwork * History of the Atom
advertisement

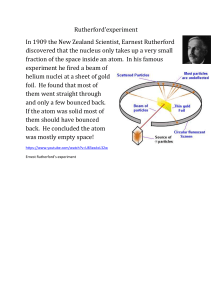
1 Chemistry Groupwork – History of the Atom Name 1. How was Rutherford able to draw his conclusions about the atom from the Gold Foil experiment? 2. What does the Law of Multiple Proportions say? Give an example (not used in notes) in your explanation. 3. Draw a labeled model of Thomson’s Plum Pudding model. What was incorrect about this model? 4. What two conclusions did Rutherford make about the atom based on his Gold Foil experiment? 5. Which of the five principles put forth by Dalton are still true today? Which are not correct? 6. How does Rutherford’s work disprove Thomson’s work? 7. Explain how Niels Bohr was able to determine that electrons orbit in energy levels based on his observations. 2 Chemistry Groupwork – History of the Atom 8. Draw a model of the atom for each of the scientists listed below: a. Dalton b. Thomson c. Rutherford d. Bohr e. Wave-Mechanical model Name