s - chem116
advertisement

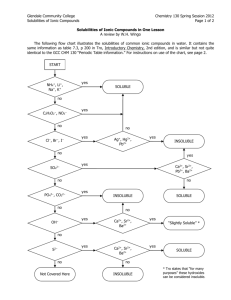
Last week… Review of ionic and net ionic equations. Review of water solubilities. Table of Water Solubilities Being able to use this table is essential for both writing ionic equations and ranking the solubility of compounds. bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Ionic and Net Ionic Equations Last week we mixed solution #2 (NaCl) with solution #4 (AgNO3). This equation is written as: NaCl + AgNO3 NaNO3 + AgCl bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Ionic and Net Ionic Equations To write an ionic equation from the given equation: NaCl + AgNO3 NaNO3 + AgCl We first need to check the table to see which compounds disassociate in water… bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Ionic and Net Ionic Equations Since the compounds in red disassociate, we need to split them up: NaCl + AgNO3 NaNO3 + AgCl becomes Na+ + Cl- + Ag+ + NO3- Na+ + NO3- + AgCl Notice the insoluble AgCl stays together. bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Ionic and Net Ionic Equations To write a net ionic equation from the newly created ionic equation: Na+ + Cl- + Ag+ + NO3- Na+ + NO3- + AgCl Simply eliminate any ion that appears on both the reactant and product side of the equation (shown in orange). This leaves us with: Ag+ + Cl- AgCl bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Table of Water Solubilities To rank solubility of compounds, simply use the table. For example, in order of increasing solubility: silver chloride (i) silver sulfate (ss) silver nitrate (s) bromide carbonate chloride hydroxide iodide nitrate oxide phosphate sulfate sulfide acetate sodium s s s s s s s d s s S silver ss i i i n i s i i ss i i= nearly insoluble ss=slightly soluble s=soluble d=decomposes n=not isolated Questions? If anything from the last two labs is still unclear, be sure to see me today BEFORE you go take the quiz! Lab #6 Stoichiometry Chemistry 108 Instructor: Kristine Cooper Stoichiometry Chemical math The branch of chemistry that quantifies the substances in a chemical reaction The art of figuring how much stuff you'll make in a chemical reaction from the amount of each reagent you start with Atomic weight Atomic weight of an element (found on the periodic table) is given in AMUs. For example, the atomic weight of carbon is 12 amu. This is the mass of ONE atom of carbon. Gram atomic weight The gram atomic weight of an element is the same NUMBER as the element’s atomic weight, the UNITS change. For example, the gram atomic weight of carbon is 12 g. This is the mass of 6.02x1023 atoms of carbon (an Avagardo’s number or one mole). Formula weight / Molecular weight Formula weight is the combined weight of all atoms in a compound. Example: – Propanol CH3(CH2)2OH What is the formula weight? Count the number of each atom, multiply by it’s atomic weight of that atom, then total. Percent composition Calculated by determining the percent (by mass) of each individual atom of the formula weight of the compound. Example: – Propanol CH3(CH2)2OH We determined the formula weight earlier to be 60 amu. Now determine what % of 60 amu the total mass of each atom comprises. Use the total mass of each type of atom determined before, then divide by the formula weight. Simplest empirical formula Example: A compound was found to contain 88.8% oxygen and 11.2% hydrogen, what is the empirical formula? 1. Set the amount of the compound as 100g. In a 100g sample, there would be 88.8g oxygen and 11.2g hydrogen. 2. Determine the moles of each kind of atom. 88.8 g O / 16 g/mol=5.55 mol O 11.2 g H / 1 g/mol = 11.1 mol H 3. Set these numbers as subscripts, then simplify the ratio (divide by the smallest subscript present). H11.1O5.5 H2O True molecular formula Simple empirical formulas only tell us the ratio of atoms present in a compound. True molecular formulas tell us the true number of each atom present. THESE ARE ALWAYS EVEN MULTIPLES OF THE EMPIRICAL FORMULA! True molecular formula For example, we determined water’s empirical formula to be H2O, which has a formula weight of 18g for each water molecule. What is the true molecular formula for 90g of water? True molecular formula First, divide 90g by the molecular weight of water (18g). 90g/18g= 5 Next, multiply each subscript in the empirical formula (H2O) by this number (5). H=2x5=10 and O=1x5=5, so the true molecular formula is H10O5. Law of Definite Composition The chemical composition of a substance is fixed, it never varies with sample size. Simply stated, water is still H2O whether you have a mL vs. 5L, etc. Stoichiometry practice Calculate the molecular weight of methane (CH4). Stoichiometry practice 12 moles of a certain compound weighs 702 g. What is the molecular weight of the compound? Stoichiometry practice Calculate the percent composition of each element in hydrogen peroxide (H2O2). Stoichiometry practice Calculate the mass (in grams) of 1.15 moles of vandium sulfide (VS2). Stoichiometry practice You want to manufacture sodium monoxide following the process: 2Na + O2 → 2Na2O. If you begin with 11.5g Na, how much O2 will be necessary to ensure all the Na has reacted? Today in Lab Given an unknown oxygen-containing potassium salt, we will determine its chemical formula. By removing the oxygen from the compound and then calculating the difference in weight with and without the oxygen, we can determine the original composition. Check out the demo compounds, compare volumes.