Ethanol Feedstock Chemistry
advertisement

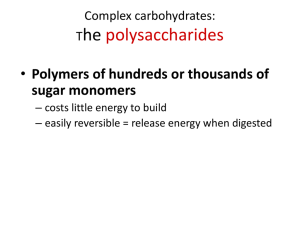
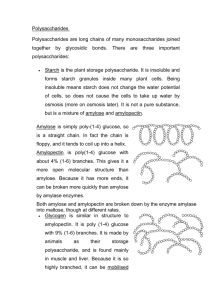
Basic Chemistry of Ethanol Production presented at CCURI Biofuels Workshop Muskegon Community College Muskegon, MI October, 17 – 20, 2013 by Chuck Crabtree Director – Iowa BioDevelopment Indian Hills Community College Ottumwa, IA Topics 1. What are carbohydrates? 2. What is glucose? 3. Why is glucose important? 4. What is starch? 5. What is cellulose? 6. How are the structures of starch and cellulose similar and how are they different? 7. How is starch used to make ethanol? 8. How is cellulose used to make ethanol? 9. What are some of the more common feedstocks used for ethanol production? Carbohydrates – Starch and Cellulose What is a carbohydrate? • Organic compounds that contain only carbon, hydrogen, and oxygen What do carbohydrates do? • Store energy (food) – starch (corn, potatoes) • Structural – cellulose (plant stems, wood) Basic component of both starch and cellulose? • Glucose Glucose Basic Structure = Oxygen = Carbon = Hydrogen Glucose Basic Structure = Oxygen = Carbon Glucose Basic Structure 6 = Oxygen = Carbon 5 1 4 3 2 Glucose Basic Structure Glucose – Important Facts • Also called “fermentable sugar,” “dextrose,” “corn sugar” or “sugar” • Used by biological systems as food • It is a “monomer” – Mono means “one” or “single.” • Starch is a “polymer” – Poly means “many.” Monomers What is a monomer? • One unit of a string of units Monomers What is a monomer? • One unit of a string of units • Example: Pearl necklace • If a monomer is 1 unit of a string of units, then what is the monomer of a pearl necklace? Monomers What is a monomer? • One unit of a string of units • Example: Pearl necklace • If a monomer is 1 unit of a string of units, then what is the monomer of a pearl necklace? • A pearl! Polymers What is a polymer? • A string of similar units Polymers What is a polymer? • A string of similar units • Example: Pearl necklace • If a polymer is a string of similar units, then what is the polymer of a pearl? Polymers What is a polymer? • A string of similar units • Example: Pearl necklace • If a polymer is a string of similar units, then what is the polymer of a pearl? • A pearl necklace! Monomers and Polymers • The pearl is a monomer – When connected with other pearls they make a polymer called a pearl necklace • Same principle applies to chemicals – Glucose is a monomer – When connected with other glucose molecules they make a polymer called starch or cellulose Starch is a polymer • > 500 glucose units • Two types of starch Starch is a polymer • > 500 glucose units • Two types of starch • Amylose – straight chains Starch is a polymer • > 500 glucose units • Two types of starch • Amylose – straight chains • Amylopectin – branched chains Starch vs. Cellulose Structure Starch Structure 6 6 6 5 5 5 4 1 3 6 4 1 3 2 5 4 1 3 2 4 2 1 3 2 3 2 Cellulose Structure 6 6 5 3 4 1 3 4 1 5 2 6 5 2 1 4 3 4 1 5 2 6 Cellulosic Structure Cellulose Structure Glucose Molecule Cellulosic Structure Cellulose Structure Individual Cellulose Molecules Glucose Molecule Cellulosic Structure Cellulose Structure Individual Cellulose Molecules Glucose Molecule Cellulosic Structure Cellulose Structure Cellulose Microfibril Lignin Individual Cellulose Molecules Glucose Molecule Cellulosic Structure Cellulose Structure Cellulose Microfibrils Lignin Individual Cellulose Molecules Non-cellulose Polysaccarides Cellulose Fiber Glucose Molecule Lignin Structure • Very complex structure • Second most common molecule on earth. • Lacks a defined structure • Fills the spaces between cellulose Hemicellulose Structure Enzymes • Enzymes are proteins that speed up chemical reactions, but are not altered themselves (catalysts) Enzymes • Enzymes are proteins that speed up chemical reactions, but are not altered themselves (catalysts) Enzymes • Enzymes are proteins that speed up chemical reactions, but are not altered themselves (catalysts) Enzymes • Enzymes are proteins that speed up chemical reactions, but are not altered themselves (catalysts) Reaction can go either way Glucose Linkages and Enzyme Active Sites Glucose Linkages and Enzyme Active Sites Glucose Linkages and Enzyme Active Sites Glucose Linkages and Enzyme Active Sites 6 5 1 4 3 2 Glucose Linkages and Enzyme Active Sites Named for the way two glucose molecules are attached to each other. 6 5 1 4 3 1,4 linkage 1,6 linkage 2 Amylopectin – Branched Starch Molecule Amylopectin – Branched Starch Molecule Introducing the enzyme α-amylase. Amylopectin – Branched Starch Molecule Introducing the enzyme α-amylase. α-amylase breaks down starch into shorter glucose chains called “dextrins.” Breaks 1,4 linkages that are not terminal. Amylopectin – Branched Starch Molecule Introducing the enzyme α-amylase. Dextrins Dextrins Dextrins α-amylase breaks down starch into shorter glucose chains called “dextrins.” Breaks 1,4 linkages that are not terminal. Dextrins Dextrins Dextrins Introducing the enzyme glucoamylase. Introducing the enzyme glucoamylase. Glucoamylase breaks down dextrins into individual glucose molecules. Acts on 1,4 and 1,6 terminal linkages. What about Cellulose Since starch and cellulose are glucose polymers, why is cellulose so much harder to break down into glucose? So how do we get through the other stuff to get to the cellulose? Once we get to the cellulose, will the same enzymes work? Cellulose Pretreatment Options Physical Breakdown (heat/pressure, grinding, chopping, sonication) Weak Caustic (NaOH, Ammonia) Weak Acid (Sulfuric, HCL) Heat with caustic and acid Pressure and heat with caustic and acid Enzymatic cocktails (cellulases, zylases, gluconases, ligases, hemicellulases, etc). Usually used after at least one of the above. Time can vary on all of these. Cellulose as Other Forms of Fuel Straight Combustion – Most common Pyrolysis (bio-oil)– Usually high temps and pressures. Some low temp technologies. Syngas – Gasification of organic material at high temps (without combustion) with oxygen and steam to produce CO, H, and CO2 Ethanol Feedstocks Feedstocks Three Types of Feedstocks • Starch-rich feedstocks • Sugar-rich feedstocks • Cellulosic feedstocks Cellulose Corn Sugar Cane Starch-Rich Feedstocks Two Types of Starch-rich Feedstocks • Tubers • Cassava • Potato • Cereal grains • Corn • Wheat • Barley • Rye • Grain Sorghum Triticale • Triticale Barley Cassava Potato Wheat Sorghum Corn Starch-Rich Feedstocks Tubers • Cassava • Potato Cassava Potato Starch-Rich Feedstocks Cereal Grains • Wheat • Barley Wheat Barley Starch-Rich Feedstocks Cereal Grains • Rye • Grain Sorghum • Triticale Rye Triticale Grain Sorghum Sugar-Rich Feedstocks Three Primary Sources • Sugar Cane • Sugar Beets • Sweet Sorghum Sugar Beets Sugar Cane Sweet Sorghum Sugar-Rich Feedstocks Different process than for starch-rich feedstocks. Sugar Feedstock Process • • • • • • • • • Sugar juice/syrup Washing preparation Breaking Milling Straining Clarification Evaporation Mash Preparation Fermentation Distillation/Dehydration Starch Feedstock Process • • • • • • • • • Milling Mashing/Cooking/Liquifaction Fermentation Distillation/Dehydration Sugar-Rich Feedstocks Sugar Cane Sugar-Rich Feedstocks Sugar Beets Sweet Sorghum Sugar Beets Sweet Sorghum Cellulosic Feedstocks Cellulose • What is it? • General term – Biomass • Straight chain polymer of glucose molecules • Used by plants in cell walls Hay Wood Chips Cellulosic Feedstocks Cellulose • Where does it come from? • Plant material • Wood • Grasses (e.g., switchgrass) • Crop residue (e.g., corn stover) Cellulosic Feedstocks Cellulose • Where does it come from? • Plant material • Wood • Grasses (e.g., switchgrass) • Crop residue (e.g., corn stover) • Advantages • No food vs. fuel issues • Worldwide distribution • High glucose density Cellulosic Feedstocks Cellulose • Where does it come from? • Plant material • Wood • Grasses (e.g., switchgrass) • Crop residue (e.g., corn stover) • Advantages • No food vs. fuel issues • Worldwide distribution • High glucose density • Disadvantages • Difficult to breakdown • Bulky Sources of Information The Alcohol Textbook, Fifth Edition, W.M Ingledew, D. R. Kelsall, G. D. Austin and C Kluhspies, eds. Nottingham University Press, Nottingham, UK, 2009. Portaria Nº 143, de 27 de Junho de 2007 (in Portuguese). Ministério da Agricultura, Pecuária e Abastecimento. Retrieved 2008-1005. This decree fixed the mandatory blend at 25% starting July 1st, 2007 Luiz A. Horta Nogueira (2004-03-22). "Perspectivas de un Programa de Biocombustibles en América Central: Proyecto Uso Sustentable de Hidrocarburos" (in Spanish) (PDF). Comisión Económica para América Latina y el Caribe (CEPAL). Retrieved 2008-05-09. UNICA: venda de veículos flex no Brasil cresce 13,9% em 2009 e frota ultrapassa 9 milhões de unidades (in Portuguese). UNICA. 2010-01-11. Retrieved 2010-02-09.