Examples
advertisement

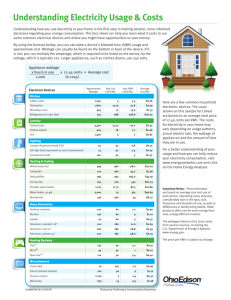
Examples • In a certain room in your house, you use a 100 W light bulb. This light is on for 5 hours every day. How much energy does it use? • 1 W = 1 J/s and there are 5h x 60min/hour x 60 sec/min = 18,000s in 5 hours so the total energy used is 100 j/s *18000s = 1.8 x 10 6 J. • Lets assume the same lighting level can be achieved using a 30 W compact florescent bulb. How much energy is used by the compact florescent bulb? Examples • Total energy = 30 j/s x 18000 s = 5.4 x 105 j. • So how much energy is saved every day using the compact florescent bulb? Take the difference between the energy used by the two different light bulbs: 1.8 x 10 6 j - 5.4 x 105 j = 1.3 x106 j. • Lets look at this in something you might be able to relate to better than joules---dollars! Example continued • After 5 hours, our 100 W light bulb uses 500 Watt-hours, or 0.5 Kwh. The 30 W bulb will use 150 Watt hours or 0.15 Kwh. • Assume electricity costs 11 cents/Kwh (average cost in the US in April 2008). So it costs .5 KwH x 11 cents/Kwh = 5.5 cents every day to run the 100 W light bulb and 0.15Kwh x 11 cents = 1.65 cents every day to run the compact florescent. Example continued • So in a year, the 100 W light bulb costs you 5.5 cents/day X 365 days/year = $20.00 and the 30 W bulb costs costs you 1.65 cents/day x 365 days/year = $5.50. Types of Energy: kinetic and potential Energy Kinetic energy energy of a moving object KE=1/2mv2 Potential Energy – Energy stored in a system, for example an object of mass m, a distance h above the surface of the earth has a potential energy given by mgh. g is the acceleration due to gravity = 9.8 m/s2 More examples of potential energy Another example is a spring, compressed a distance x from its equilibrium point has a potential energy 1/2kx2, where k is the spring constant, a property of the spring. Chemical Energy Energy that is released via chemical reactions. Often times release is through combustion such as energy generation via coal Another example is a battery Heat Energy Energy associated with the random motions of the molecules in a medium. Measured by temperature • Temperature Scales: • Fahrenheit – based on the height of liquid (often mercury or alcohol) in a glass tube. • Celsius – another scale using height of liquid in a tube • Kelvin-absolute scale – True measure of energy Fahrenheit temperature scale • Freezing point of water set at 32 and boiling point set at 212, so there is 180 degrees between them and each degree is 1/180 of the difference between these two points. Celsius temperature scale • Freezing point of water set at 0 and boiling point set at 100, so there is 100 degrees between them and each degree is 1/100 of the difference between these two points. Kelvin temperature scale • O k is absolute zero. All molecular motion stops. • Interval set so that 1 k = 1 c • So to convert from c to k k=c+273 Mass Energy • E = mc2 • Energy and mass are equivalent • C = 3 x 108 m/s. • A big number and its squared! So even if m is small, E is big. • A small mass, converted to energy, gives a lot of energy! Example Electromagnetic energy •Light displays properties of both waves and particles. •Light is an electromagnetic wave-a wave created by alternating electric and magnetic fields. •“Light” is more than just visible light, it covers wavelengths from radio thru Gamma rays •Light is also a “particle” called a photon. •Photons have energy given by E=hν or E=hc/λ. H is constant, c is the speed of light , ν is the frequency of light and λ is the wavelength of the light. Conservation of Energy • The principle of conservation of energy states that energy cannot be created or destroyed. But it can be converted form one form to another • This idea of energy transformation is at the heart of energy generation. Energy Sources renewable vs nonrenewable • Renewable – can’t be exhausted • Solar • Geo-thermal • Tidal • Wind • Hydro • Non-renewable-can be exhausted • Fossil fuels (oil, coal etc) uranium How much do we use? • World energy consumption • US energy consumption How much do we use? How much do we use? • Almost 95% of the energy we use comes from non-renewable energy sources! • One of these days we will run out, and then what? • What are some short and long term answers to this question?