Higher Unit 1
advertisement

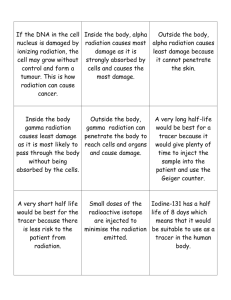
In unit 4 we will learn about energy from the nucleus and its applications. * What do you know? How do we get energy from the nucleus? What do we mean by energy? What do we mean by nucleus? What do we use it for? What do we know? * Ionising Radiations are used in many medical applications including X-rays and sterilising hospital equipment. They are also used in many non medical applications and it is important in many fields of work to understand radiation dose and safety. Nuclear reactors are used in the production of around 11% of the world’s energy production, and to power some military ships and submarines. Key words: atom, protons, neutrons, electrons, radiation energy, absorption, alpha, beta, gamma, ionisation By the end of this lesson you will be able to: Describe a simple model of the atom which includes protons, neutrons and electrons. State that radiation energy may be absorbed in the medium through which is passes. State the range through air and absorption of alpha, beta and gamma radiation. Explain what is meant by an alpha particle, beta particle and gamma radiation. Explain the term ionisation. State that alpha particles produce much greater ionisation density than beta particles or gamma rays. * Useful Radiation Radiation has many uses in medical Physics – different types of radiation are used for different things. Baggage scanning Smoke Detectors Smoke alarms contain a weak source made of Americium-241. Alpha particles are emitted from here, which ionise the air, so that the air conducts electricity and a small current flows. If smoke enters the alarm, this absorbs the alpha particles, the current reduces, and the alarm sounds. Am-241 has a half-life of 460 years. Radioactive Dating Animals and plants have a known proportion of Carbon-14 (a radioisotope of Carbon) in their tissues. When they die they stop taking Carbon in, then the amount of Carbon-14 goes down at a known rate (Carbon-14 has a half-life of 5700 years). The age of the ancient organic materials can be found by measuring the amount of Carbon-14 that is left. Leaking Pipes Radioactivity is used in industry to detect leaks in pipes. To have a good understanding of radioactivity we need to know a bit about the structure of the atom What do atoms look like? They are very small! Atoms are the smallest possible particles of the elements which make up everything around us Structure of the atom nucleus proton neutron electrons Structure of the atom nucleus proton neutron electrons The relative masses and charges of these particles are given below PARTICLE CHARGE MASS Proton +1 1 Neutron 0 1 Electron -1 1/ 2000 Relative size of the atom and the nucleus. The ratio of the diameters is 10 000 : 1 ! If the diameter of a particular atom was 10 metres, its nucleus would be 1 millimetre across!! The atoms of a particular element are identical: All carbon atoms have 6 protons in the nucleus and 6 orbiting electrons. * Atoms usually have the same number of protons and electrons so an atom has no overall charge. Six protons – charge? +6 Six electrons – charge? -6 Overall charge? 0 Ionisation We will learn about types of radiation which cause ionisation. Ionisation Ionisation means adding or removing an electron from an atom to produce a charged particle. What happens to the charge on an atom when an electron is added or removed? Atoms contain protons, which are positive as well as electrons, which are negative Normally atoms have equal numbers of protons and electrons and are therefore neutral Atoms usually have the same number of protons and electrons so an atom has no overall charge. Six protons – charge? +6 Six electrons – charge? -6 Overall charge? 0 If you add an electron… Six protons – charge? +6 Six electrons – charge? -6 Add one more electron – charge? Overall charge? -1 -1 If you remove an electron… Six protons – charge? +6 Six electrons – charge? -6 Take away an electron – charge? Overall charge? +1 -5 Ionisation means the addition or removal of an electron from a neutral atom to produce a charged particle. Virtual Int 2 Physics -> Radioactivity -> Ionising Radiations -> Model of the Atom * The picture below shows an ALPHA PARTICLE, consisting of 2 protons and 2 neutrons electron Imagine that an ALPHA PARTICLE passes through a neutral atom – this will be shown in slow motion! An electron has been knocked out of the atom. This atom is now positively charged – it is a POSITIVE ION. There are three types of ionising radiation. Alpha radiation (a) Beta radiation (β) Gamma radiation (γ) Virtual Physics Int 2 – Radioactivity -> Ionising Radiations -> Alpha, Beta, Gamma Alpha radiation (α) An alpha particle is made up of two protons and two neutrons. It is the same as a helium nucleus. 4 2 He It is positively charged. It is largest of all the three types of radiation. A big atom releases an alpha particle to make itself more stable. The alpha particle that is emitted has a lot of energy and can damage human cells. * Alpha radiation (α) An alpha particle is given the symbol 4 2 a a He 4 2 * Summary What are An alpha particle is alpha made up of two particles? protons and two neutrons. It is the largest of the three ionising radiations. It has a lot of energy. * Beta radiation (β) a fast moving high energy electron released from the nucleus – it is very very small Virtual Physics Int 2 – Radioactivity -> Ionising Radiations -> Alpha, Beta, Gamma * Beta radiation (β) A beta particle is given the symbol or 0 1 This is what happens inside the nucleus. * Summary What are A beta particle is a beta fast moving, high particles? energy electron. The electron is released from the nucleus when a neutron changes into a proton plus electron. It is very very small. * Gamma Radiation (γ) A wave of energy. High frequency electromagnetic wave (so travels at the speed of light) No significant mass. No charge. Has the greatest amount of kinetic energy. * Gamma ray It is the most energetic of all three radiations. It is therefore the most penetrating – the most difficult to stop. * What are Gamma rays are gamma high energy rays? electromagnetic waves. They travel at the speed of light. * Radiation & Ionisation These three radiations (α, β, γ) are called ionising radiations because they cause ionisation of living cells. Radiations can kill or change living cells. This is what makes them dangerous. Ionisation Density We can think about how much damage a type of radiation will cause in terms of ionisation density. Alpha particles are heavy and slow moving. They cause a lot of ionisation. Beta particles are light and cause less ionisation. Gamma rays have no mass. They cause little ionisation. * ALPHA PARTICLES are relatively large and cause a lot of ionisation +- + + - + + - + - + - - ++ - + + - + BETA PARTICLES are smaller, so they cause less ionisation - + + - + - + GAMMA RAYS cause least ionisation of all + Ionisation Density & Range of Particles Each time a particle causes ionisation it loses energy. The energy is absorbed by the medium through which it passes. Alpha particles cause a lot of ionisation, therefore lose a lot of energy. This means they have a short range in air. Ionisation Density & Range of Particles Beta particles cause less ionisation, therefore lose less energy. This means they have a longer range in air than alpha particles. Gamma particles have the lowest ionisation density. This means they have the longest range in air. Identifying Radiations We can tell which radiation is which by testing to see what happens when they reach different materials. Virtual Int 2 – Radioactivity -> Ionising Radiations -> Absorption of Ionising Radiations What material is sufficient to absorb alpha particles? Paper What material is sufficient to absorb beta particles? A few millimetres of aluminium What material is sufficient to absorb gamma rays? Several cm of lead How much ionisation do alpha particles cause? The greatest amount. Alpha particles are most dangerous when inside the body (but least dangerous outside – they can be stopped with paper!) How much ionisation do beta particles cause? Medium. Less than alpha, more than gamma. How much ionisation do gamma particles cause? The least. Gamma particles are most dangerous when outside the body because they can easily travel into the body. But they’re least dangerous when inside because they can escape. Can you…? Describe a simple model of the atom which includes protons, neutrons and electrons. State that radiation energy may be absorbed in the medium through which is passes. State the range through air and absorption of alpha, beta and gamma radiation. Explain what is meant by an alpha particle, beta particle and gamma radiation. Explain the term ionisation. State that alpha particles produce much greater ionisation density that beta particles or gamma rays. Quick Recap Type of radiation Symbol What is this Charge and radiation? absorption Range in air α A few m Uncharged. Absorbed by lead. Key words: atom, protons, neutrons, electrons, radiation energy, absorption, alpha, beta, gamma, ionisation By the end of this lesson you will be able to: Describe how one of the effects of radiation is used in a detector of radiation. State that radiation can kill living cells or change the nature of living cells. Describe one medical use of radiation based on the fact that radiation can destroy cells. Describe one use of radiation based on the fact that radiation is easy to detect. Detecting Radiation To protect those who work with radiation it is important to be able to detect radiation. The detection of radiation is also vital in its use in many applications. Geiger Muller Tube The Geiger counter is commonly used to detect radiation (demo). The Geiger counter consists of a Geiger Muller tube attached to a counter. Geiger Muller Tube The tube is filled with argon gas. Where else is argon gas used? Geiger Muller Tube Around 400 V is applied to the thin wire. Geiger Muller Tube Radiation causes ionisation of the gas – what do we mean by this? The thin window alllows radiation to enter. Geiger Muller Tube Ions produce electrical pulses which are counted and displayed. Geiger Muller Tube We can either display total counts and use a timer to determine counts per second, or use a rate meter, which displays counts per second. Geiger Muller Tube Radiation Ionisation in tube (lots of electrons) Discharges central wire Counted as a pulse * How the Geiger Muller tube works Photographic Fogging We know that photographic film can be fogged or blackened by radiation. Where is this commonly used in medicine? Photographic Fogging This principle is used in film badges worn by radiation workers. The darker the film the more radiation the person has received. Photographic Fogging Why are there different materials in the film badge? Photographic Fogging Different radiations pass through or are absorbed by different materials. * Radiation and the Human Body When the source of radiation is outside the body, alpha radiation may not be able to harm the vital internal organs as it is easily stopped by the air, layers of clothing or the skin. If swallowed an alpha radiation source is extremely dangerous. It causes large amounts of ionisation (remember it has a high ionisation density) – it changes or kills a lot of living cells. It can’t escape from the body. Alexander Litvinenko Poisoned using extremely rare radioactive substance Polonium-210 – which is 250000 more toxic than hydrogen cyanide. Swallowing a dose less than 1/10th the size of a Smartie is lethal for a grown adult male. Radiation and the Human Body Beta radiation will penetrate the first 1cm or skin and tissue though, and will damage that tissue. A small amount can penetrate the body. If the beta source is inside the body, then it will cause damage internally, for example to organs. Radiation and the Human Body Gamma radiation will penetrate the skin and tissue, and will deposit its energy as it travels further into the body. It is more dangerous than alpha or beta radiation in this case. Gamma radiation inside the body will also damage tissue however it can “escape” and be detected from outside the body, and this makes it very useful. Making Use of Radioactivity Gamma radiation’s ability to travel through skin and tissue is used in medical and non medical applications of radioactivity. The gamma camera Radioactive Tracers A radioactive tracer is a gamma emitting substance (a radiopharmaceutical) which can be injected into the body to allow internal organs and functions to be investigated without surgery. Radioactive Tracers Technetium-99 and Iodine-123 are commonly used because they emit only gamma, which can be detected outside the body, and cause little ionisation. However, different substances are chosen for different organs. Radioactive Tracers A gamma camera is used to detect radiation from outside the body. This scan is produced after a few hours of the patient being injected with an isotope that emits gamma radiation. A detector is moved around the body and a computer produces an image. Dark areas show high concentrations of radiation coming from those parts. This indicates increased blood flow to these parts. If a radioisotope that emits alpha radiation is used, no particles can be detected outside the body – why not? Alpha radiation will be stopped within a few centimetres. Internal organs will be seriously damaged. Isotopes that emit gamma radiation must be used – why? Since gamma rays will pass through the body (and out) while doing the least damage. Radioactive Tracers in Industry Leaks in underground pipes can be detected using radioactive tracers and a Geiger Counter. A rise in count rate detected would indicate more radiation escaping the pipe and therefore a leak or crack. Oil companies also use radioactive tracers in shared pipelines to identify their own oil. Radiation Therapy Radiotherapy is commonly used as part of treatment for cancer. It might be used instead of surgery, or after surgery, or chemotherapy, to destroy any remaining cancer cells. Treating Cancer (Radiotherapy) Ionising radiation kills living cells. Cancers are simply growths of cells which are out of control and have formed tumours. By directing radiation at the tumour, the living cells are damaged or killed, and this shrinks the tumour. Unfortunately healthy cells are also damaged or killed by the radiation. Treating Cancer (Radiotherapy) It is important to ensure that healthy tissue does not receive too much radiation while the tumour receives enough to damage it. Treating Cancer (Radiotherapy) Video clips. http://www.ccotrust.nhs.uk/about/sitemap/ac cess_map.htm The machine rotates around the patient. The tumour can be hit by radiation all of the time while minimising the damage to healthy tissue. Each section of healthy tissue receives only a small dose. Treating Cancer (Radiotherapy) Why are alpha and beta sources unsuitable for radiotherapy treatments? Alpha and beta are absorbed by air/skin/bone so would not reach the diseased tissue within the body. Instead high energy X-rays are used. * Radiation & Sterilisation The ability of radiation to kill living cells makes it very useful for sterilising equipment e.g. plastic syringes in hospital. Previously expensive metal or glass syringes had to be used and sterilised using heat or chemicals. Using heat to kill germs and bacteria would melt the plastic syringes. Paper Thickness Measurement in Industry Virtual Int 2 Physics -> Radioactivity -> Ionising Radiations -> Uses of Ionising Radiations A beta source and detector is used. If the paper is too thin then the reading on the detector will increase. If it is too thick, the reading will decrease. Why is an alpha source no use for this application? Key words: activity, radioactive source, decays, decays per second, becquerels, absorbed dose, grays, radiation weighting factor, equivalent dose, background radiation level By the end of this lesson you will be able to: State that the activity of a radioactive source is the number of decays per second and is measured in becquerels (Bq), where one becquerel is one decay per second. Carry out calculations involving the relationship between activity, number of decays and time. State that the absorbed dose is the energy absorbed per unit mass of the absorbing material. State that the gray (Gy) is the unit of absorbed dose and that one gray is one joule per kilogram. By the end of this lesson you will be able to: State that a radiation weighting factor is given to each kind of radiation as a measure of its biological effect. State that the equivalent dose is the product of absorbed dose and radiation weighting factor and is measured in sieverts (Sv). Carry out calculations involving the relationship between equivalent dose, absorbed dose and radiation weighting factors. By the end of this lesson you will be able to: State that the risk of biological harm from an exposure to radiation depends on: a) the absorbed dose b) the kind of radiation, e.g. α, β, γ, slow neutron c) the body organs or tissue exposed. Describe factors affecting the background radiation level. How much exposure is safe? It should be stressed that no minimum amount of exposure to radiation is completely safe. In Physics we aim to understand how to measure radiation and to estimate the risk of exposure. In many cases the benefit of exposure significantly outweighs the risks. Radioactive Decay Radiation is caused by the unstable nucleii of radioactive atoms splitting up. This is called radioactive Virtual Int 2 Physics -> Radioactivity -> Dosimetry -> Activity decay. Activity We talk about the activity of a source. What do we mean by this? The activity of a radioactive source is a measure of the number of decays per second. Units of Activity The becquerel is used to measure the activity of a source. 1 becquerel (Bq) is one decay per second. Activity Number of nuclei decaying N A t Activity (Bq) Time (s) The becquerel In practice, particularly in medical treatment, the Bq is too small. Larger units such as kBq and MBq are commonly used. Dosimetry: Absorbed Dose When radiation reaches the body or tissue it is absorbed. This is called the absorbed dose (D). Dosimetry: Absorbed Dose Energy (J) E D m Absorbed dose – units? Mass (kg) Dosimetry ABSORBED DOSE (D) is the energy absorbed PER UNIT MASS of absorbing tissue. E D m Units are GRAYS (Gy) 1 Gy = 1 J/kg Dosimetry Radiation Treatment Absorbed dose (Gy) Chest X-ray 0.00015 CT Scan 0.05 Gamma rays which would just produce reddening of skin 3.0 Dose which if given to whole body in a short period would prove fatal in half the cases 5.0 Typical dose to a tumour over a six week period 60.0 Biological Harm from Radiation Radiation can damage living cells through heat or damage to molecule structure such as DNA. The risk of biological harm from an exposure to radiation depends on • the absorbed dose • the type of radiation (e.g. alpha, or other nuclear particles such as neutrons) • the body organs or type of tissue EQUIVALENT DOSE (H) is a quantity which takes into account the TYPE OF RADIATION. H DWR WR is the WEIGHTING FACTOR of the particular radiation Unit of equivalent dose is sieverts (Sv) Typical Equivalent Dose Investigation Equivalent dose (mSv) Chest X-ray 0.1 Spine X-ray 2.0 Stomach X-ray 4.0 CT Scan 1 to 3.5 Bone Scan 2.0 Annual exposure of aircraft crew 2.0 Renogram 2.0 Astronaut in space for one month 15.0 How much is a sievert (Sv)? If 100 people received a dose of 1 Sv, 4 would die as a result. This is the type of dose you’d receive after a nuclear accident. 1 We normally work in millisieverts (mSv = Sv ) 1000 6 or microsieverts (μSv = x10 Sv) 1 mSv = One thousandth of a sievert = 0.001 Sv 1 μSv = 0.000001 Sv Example A 50kg person is exposed to radiation of energy 0.25J. The weighting factor for the radiation is 20. (a) Calculate the absorbed dose for this radiation (b) What is the equivalent dose? Example (a) Calculate the absorbed dose for this radiation E 0.25 D 0.005Gy m 50 Example (b) What is the equivalent dose? H DWR 0.005x20 0.1Sv or 100mSv 1 mSv is about 100 times the radiation you experience when you travel by aircraft on holiday. If you are part of the aircrew, you will experience larger amounts due to the amount of travel. There are regulations about total flying times which take into account exposure to radiation. In the UK people receive an average of 2 mSv each year from background sources (cosmic rays, radon gas etc). Legal limits have been set on the additional dose equivalent which people can receive: Members of the public – an additional 5 mSv each year Workers exposed to radioactivity an additional 50 mSv each year Background Radiation Life on Earth has evolved to cope with this. Your cells have selfrepairing mechanisms which allow them to survive relatively unscathed. The amount of background radiation varies considerably around Britain, as shown on the map. You can see that it is particularly high in Cornwall, because of the types of rock there. Background Radiation Background radiation is present all around us from natural and artificial sources. Sources which contribute to background radiation are: radon from rocks and soil Chernobyl and fall out from weapons testing medical uses of radiation gamma rays from building materials cosmic radiation from outer space industrial use nuclear industry Chernobyl (April 1986) Failure in safety procedures meant nuclear reaction became out of control 30 people died immediately, a Further 19 within four months. 135000 were evacuated from their homes in a 20 mile radius. Long term consequences Thyroid cancer increased ten fold with biggest increases in children under 15. Difficult to assess – and much controversy. Key words: activity, radioactive source, half life, shielding, safety precautions By the end of this lesson you will be able to: State that the activity of a radioactive source decreases with time. State the meaning of the term ‘half-life’. Describe the principles of a method for measuring the half-life of a radioactive source. Carry out calculations to find the half-life of a radioactive isotope from appropriate data Describe the safety procedures necessary when handling radioactive substances. State that the dose equivalent is reduced by shielding, by limiting the time of exposure or by increasing the distance from a source. Identify the radioactive hazard sign and state where it should be displayed. Half-Life Each radioactive substance has a different half-life. The half life is the time taken for half the radioactive nuclei to disintegrate OR the time taken for the activity of a source to fall by one half. Radioactive Decay and Half Life The activity of a radioactive source decreases with time. Virtual Int 2 Physics – Radioactivity – Half Life Radioactive Decay and Half Life The graph of activity (measured in counts per second) against time has a distinct shape. Virtual Int 2 Physics – Radioactivity – Half Life Radioactive Decay & Half Life Activity (Bq)) Sketch a graph of activity against time Time (s) Finding the half life of a source We can find the half life of a radioactive source but we must remember to correct for background radiation. If we are measuring the activity of a source we must always take off the Radiation background radiation Background For example: We measure background radiation at 2 counts each second. We then introduce a source and find that there are 47 counts each second. What is the radiation due to the source? Source radiation = total radiation – background radiation Source radiation = 47 – 2 = 45 counts each second. Draw out this table Time (s) Counts per second Corrected count rate 0 10 20 30 40 Continue to 250 seconds Measuring Background Radiation Counts in 60 seconds = Counts per second = Tasks Use the data to plot a graph of corrected count rate against time. Remember to label axes and include units. Calculate 2 or 3 half life values from the graph and find the average half life. What makes a good graph? Measuring the Half-Life of a radioactive source Activity (Bq)) Read the time taken for the activity to half. You can choose any starting point. The half life is found by calculating T2-T1. T1 T2 Time (s) Activity (Bq)) Measuring the Half-Life of a radioactive source T1 T2 Time (s) Construct a table like this: 2nd 1st activity activity 80 40 70 35 60 30 T1 (s) T2 (s) Average half-life = ……………. s Half-life (s) Radioactive Decay and Half Life Half Life Calculations Below is a graph of corrected count rate plotted against time Corrected Count Rate (counts/sec) 1200 In this case the half-life is 10 minutes. 600 10 Time (min) Time elapsed (mins) Count rate (counts/sec) Fraction of initial count rate 0 1200 1 10 600 ½ 20 300 ¼ 30 150 1/8 40 75 1/16 50 37.5 1/32 Half Life Calculations A freshly prepared radioactive substance has an initial activity of 60kBq. What will its activity be after 1 hour if the half life is 15 minutes? 1 hour = 4 x 15 minutes So the substance has been through ? half lives 4 Half Life Calculations After 1 half life the activity falls by half From 60 kBq to ? kBq. 30 After 2 half lives, the activity halves again From 30 kBq to ? kBq. 15 Half Life Calculations After 3 half lives the activity halves again From 15 kBq to ? kBq. 7.5 After 4 half lives, the activity halves again From 7.5 kBq to ? kBq. 3.75 Half Life Calculations A radioactive sample has an initial activity of 800 Bq. What is the substance’s half-life if the activity takes 24 years to decrease to 100 Bq? Initial activity = 800 Bq After 1 half life = 400 Bq After 2 half lives = 200 Bq After 3 half lives = 100 Bq so in 24 years the substance has gone through 3 half lives. 3 half lives in 24 years 1 half life in 24/3 = 8 years. Radiation Safety Protection when using radiation There are three methods by which radiation exposure can be reduced: 1. Shielding a source with an appropriate thickness of absorber e.g. a radiographer wears a lead lined apron e.g. radioactive sources are stored in lead containers. Protection when using radiation There are three methods by which radiation exposure can be reduced: 2. Limiting the time of exposure e.g. sources should be moved and used as quickly as possible Protection when using radiation There are three methods by which radiation exposure can be reduced: 3. Distance from source The further you are from the source the less radiation you will receive. In fact, if you double the distance you will receive only a quarter of the radiation. Radiation Safety What safety precautions should be taken when working with radioactive sources? Radiation Safety Use forceps or a lifting tool to remove a source – never bare hands. Keep radiation window away from the body. Never bring a source close to your eyes. After any experiment with radioactivity, wash hands thoroughly. Radiation Safety The symbol for radiation sources being stored must be displayed where radiation is being used or stored. It is an international symbol which can be seen in hospitals, schools, colleges and in industry. The Biological Effects of Radiation The amount of damage caused depends on: 1. 2. 3. the absorbed dose the kind of radiation the body organs or tissue exposed to the radiation. The biological risk caused by radiation is represented by the equivalent dose measured in sieverts (Sv). Questions 1. What is meant by ionisation? 2. (a) Why is ionising radiation dangerous. (b) When is ionising radiation produced? (c) Which is the most ionising of the three types of radiation? (d) Why is alpha radiation not dangerous if the source is outside the body? (e) Why is alpha radiation the most dangerous if the source is inside the body? 3. (a) Why is it possible to use photographic film to detect ionising radiation? (b) Explain how a film badge works. (c) How can fluorescent materials be used to detect ionising radiation? 4. A radioactive source gives out one type of radiation. A Geiger-Muller tube and counter are used in an experiment to determine the radiation present. The detector is placed directly above the source and the count rate measured with different substances between the detector and the source. (a) What correction must be made to the count rate before it can be used to determine the type of radiation present ? (b) The corrected count rate does not fall significantly when a sheet of paper is placed between the source and detector, however, it falls to the background level when a sheet of aluminium is used. Identify the radiation and explain the reason for your choice. Key words: nuclear reactors, chain reaction, fission, fuel rods, moderator, control rods, containment vessel, coolant, nuclear waste. By the end of this lesson you will be able to: State the advantages and disadvantages of using nuclear power for the generation of electricity. Describe in simple terms the process of fission. Explain in simple terms a chain reaction. Describe the principles of the operation of a nuclear reactor in terms of fuel rods, moderator, control rods, coolant and containment vessel. Describe the problems associated with the disposal and storage of radioactive waste. Nuclear Power Is nuclear power renewable or nonrenewable? Strictly non-renewable because the uranium fuel is a finite resource. At the current rate of use the existing reserves will last a long time. The ‘spent’ fuel can be re-processed and used again. Nuclear Power – What are the advantages? A lot of energy is produced per kilogram of uranium. - 1 kilogram of coal produces 30 million Joules 30 x 106 J or 30 MJ - A kilogram of uranium produces 5 million million Joules 5 x 1012 J of energy. Nuclear Power – What are the advantages? Nuclear power plants generate relatively little carbon dioxide so contribute little to global warming. Technology is readily available and well established. It is reliable. Large amount of electricity can be generated by one plant. Produces small amount of waste. Nuclear Power – do we rely on it? In the UK, about 50% of energy is created from nuclear sources. In France it is about 70%. Nuclear Power – What are the disadvantages? Nuclear power stations produce radioactive waste – which can be harmful to us and the environment. The waste must be stored safely for many years – sealed and buried. Nuclear Power – What are the disadvantages? Chernobyl demonstrated the risks of this type of technology. Nuclear power is reliable, but a lot of money has to be spent on safety - if it does go wrong, a nuclear accident can be a major disaster. People are increasingly concerned about this - in the 1990s nuclear power was the fastest growing source of power in much of the world. In 2005 it was the second slowest-growing. There are 3 main types of power station: THERMAL POWER STATION NUCLEAR POWER STATION HYDROELECTRIC POWER STATION Each type has the same basic plan Thermal Coal is burned chemical energy to heat Hydro-electric Water behind dam potential energy to kinetic Turbine kinetic energy Generator kinetic to electrical energy Nuclear Nuclear reaction nuclear energy to heat 1. Coal stockpile 2. Pulveriser which breaks the coal down – why is the coal broken up before use? 3. Boiler – coal is burnt to produce heat energy, the heat boils the water to produce steam. The steam is used to turn the turbines 4. Turbines – these have hundreds of blades. The steam from the boiler hits the blades and turns the turbine. The turbine has a shaft attached to it. As the turbine turns so does the shaft. The shaft from the turbine is connected to the generator. 5. Generator 5. Generator – the generator is made up of large electromagnet and coils of wire. The electromagnet is attached to the shaft from the turbine and turns inside the wire coils. As the electromagnet turns an electrical current is produced in the coil of wire. 6. Transformer 6. Transformer – the transformer increases the voltage of the electricity from 20 000 V to 275 000 V. This allows the electricity to be transported efficiently through the electrical transmission system. 7. Cooling Tower – after the steam has turned the turbine it is piped to the condenser. Cold water is pumped from the cooling towers where it is used to cool the steam. After circulating round the condenser the cooling water which is now about 10 ºC warmer, flows back to the cooling tower. The water is cooled by air and then falls back down to the bottom of the cooling tower to be recycled through the condenser (8) again. Some of the heat from the water is released into the air in the form of water vapour which you can see coming out of the top of the tower. 7. Cooling Tower – after the steam has turned the turbine it is piped to the condenser. Cold water is pumped from the cooling towers where it is used to cool the steam. After circulating round the condenser the cooling water which is now about 10 ºC warmer, flows back to the cooling tower. The water is cooled by air and then falls back down to the bottom of the cooling tower to be recycled through the condenser (8) again. Some of the heat from the water is released into the air in the form of water vapour which you can see coming out of the top of the tower. Stages In A Coal-Fired Power Station 1. 2. 3. 4. 5. 6. 7. 8. Coal stockpile Pulveriser Furnace & Boiler Turbines Generator Transformer & National Grid Cooling Tower Condenser A Conventional (Fossil Fuel) Power Station Energy Changes Energy Efficiency What do we mean by the efficiency of a machine? How can we write this as an equation? Energy Efficiency useful energy out efficiency x 100 total energy input Units? Why is the useful energy out always less than the total energy input? Efficiency It can be useful to consider the energy each second rather than total energy. What would the equation be for efficiency using energy each second? power out efficiency x 100 power in Efficiency (as a percentage) useful energy out % efficiency x 100 total energy input The efficiency of a power station (or any machine) tells us how much of the input energy is converted to useful output energy. Energy that is LOST has been converted to less useful forms such as heat. Efficiency Fuel Consumption To determine the amount of fuel required: total energy required Number of kilograms of fuel energy stored in each kg of fuel Note that power is energy each second so for a given power output we can find the fuel needed each second. Nuclear Power Like fossil fuels, uranium is mined. A lengthy (and expensive) process is required to extract the uranium from the ore. Inside the Nuclear Power Station http://science.howstuffworks.com/nuclear-power2.htm Inside the Nuclear Power Station In place of the boiler found in a conventional power station, there is a reactor. Heat energy produced during nuclear fission is carried by carbon dioxide gas to a heat exchanger where it heats water, turning it into steam. The steam drives a generator to produce electrical energy. The steam is cooled (turned back into water) and pumped round for reuse. Inside the Reactor To obtain energy from uranium-235 nuclei, they are bombarded with neutrons. (What is a neutron?) The neutron is absorbed by the uranium-235 nucleus making is unstable – it splits into two pieces releasing a large amount of (heat) energy and two further neutrons. This process is called fission. http://library.thinkquest.org/26285/english/animation.html Chain Reaction The two neutrons released then strike two further uranium nuclei. This time four new neutrons are produced which cause further fissions, producing more neutrons and so on. This continuous reaction of fissions is called a chain reaction. http://www.npp.hu/mukodes/anim/Uuu13-e.htm http://www.npp.hu/mukodes/anim/div2a-e.htm Nuclear Fission The total mass at the end is less than the mass at the start. The lost mass has changed into energy - E = mc2 m = the loss in mass and c = speed of light A Chain Reaction A Chain Reaction The 2 neutrons released during the nuclear fission can go on to bombard further uranium nucleii which causes further nuclear fission releasing even more neutrons which can in turn go on to produce more fission An uncontrolled chain reaction is used in a nuclear bomb In a nuclear power station the rate of reaction is controlled using boron control rods which can be lowered into the reactor and absorb the neutrons that induce the fission process. The fuel rods These rods contain natural uranium which is enriched so that fission can occur. The amount of uranium in a fuel rod is well below critical mass so that an explosion cannot naturally occur. Fuel rods have to be replaced every few years. The Graphite Moderator When the neutrons are emitted after fission they are moving very fast. They will not be able to be “captured” by other nuclei so fission will not occur. If they are slowed down there is a greater chance that fission will occur. This is done using a graphite moderator – collisions with graphite atoms slow the neutrons down. Keeping the Chain Reaction under control http://www.npp.hu/mukodes/anim/sta1e.htm The Control Rods The amount of electrical power required will vary with peak demand during the day and lower demand at night. Rods of boron absorb additional neutrons and control the number available for fission. They can be raised and lowered as necessary, and provide an important safety feature. In the event of an accident, all rods are lowered to absorb neutrons and stop the chain reaction. Coolant The heat produced during the reaction must be removed from the reactor. This is done using the coolant – normally carbon dioxide. The carbon dioxide is continually heat, then passes the heat to water via the heat exchanger. The water turns to steam, which drives the turbine. Calculating amount of fuel required for power output To determine the amount of fuel required to produce a given power output Number of kg of fuel = total energy required energy stored in each kg of fuel Energy Changes in a Nuclear Power Station Note that in conventional fossil fuel power stations AND in nuclear power stations the energy source is used to raise steam to drive turbines to drive the electricity generator. Containment Vessel The key parts of the nuclear reactor which form the core, are contained in a containment vessel. This is designed so that no radiation can escape – it is several metres thick and has a concrete top. Disposal of Nuclear Waste There are different categories of nuclear waste. High level – mainly spent nuclear fuel. After several years of use fuel rods are taken out and sent for reprocessing – removal of useful parts which can be made into new rods. Disposal of Nuclear Waste High level – unfortunately what remains after reprocessing is highly radioactive. Storage is initially in water for around a year before the waste can be handled. However, it has a very long half life, remains extremely dangerous and there is as yet no ideal solution for long term safe storage. Disposal of Nuclear Waste Low level – this is, for example, waste generated by hospitals etc. It is still dangerous and must still be stored. It used to be dumped at sea but this is now banned. With either type of waste the problems are - storage methods - storage sites – including transportation