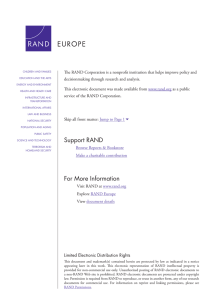
Ethics Appraisal Procedure – in 4 steps
advertisement

H2020 Ethics Ethics Appraisal Procedure - Guidance and examples Horizon 2020 and Ethics Ethics is an integral part of all research activities funded by the European Union, from beginning to end at all levels.– reference http://ec.europa.eu/research/participants/docs/h2020-fundingguide/cross-cutting-issues/ethics_en.htm The broad topic of ethics in research is considered from different aspects: Research integrity „Ethics-ready“ proposals Ethics monitoring during the implementation of projects Funding of ethics research Responsible research and innovation (RRI) Pointers ! Research ethics is not a one size fits all approach ! It is strongly recommended, when planning your project, that you give yourself enough time in case it needs to go through screening, assessments and check/audits. ! The Ethics Paragrapgh is not part of the scientific evaluations, but will be evaluated separately later Ethical Table Self assessment. Self Assessment New developments • EU regulation for Protection of Privacy • Adoption Mid June • Will have effect on Research esp. concerning collection personal data and safety of personal data. • University Data Protection Officer will become Obligatory Box 1 en 3 • Box 1. Human Embryos/ Foetuses • Box 3. Human Cells and Tissues • Ethical Committee Medical Research (METC) (LUMC) Contact: cme@lumc.nl +31 (0)71- 52 63241 / 66963 Box 2 en 4 • • • • Box 2. Humans • A. Psychology in combi with human cells and tissues: METC • B. Human volunteers is SS&H research Ethical Approval if applicable ( unable to give consent; vulnerable people; children; patients; ) Box 4: protection of Personal data • Collection en processing personal data; genetic information; tracking & observation; secondary use of PD Beschrijven van procedures of informed consent en voorbeelden van te gebruiken forms. Binnen faculteiten bestaan voorbeelden. Approval for collecting /processing Personal data: – Tot de adoptie van de nieuwe europese regelgeving: NL wetgeving van kracht : melding bij Collge Bescherming persoonsgegevens. – Na de adoptie van de europese regelgeving: GEEN NL wetgeving meer maar EU: universiteit is verplicht een DPO aan te stellen . Dit wordt een toezichthouder – Beschrijving van de procedures met behulp van UB. Box 5 en 7 • Box 5 : Dieren – Wet op de dier proeven – DEC voert ethics toetsing – Information on Animal Welfare http://www.science.leidenuniv.nl/index.php/faculteit/diergebonden_onderzoek/onderzoekslijn en • Box 7: Environment & Health and Safety – Kaderregeling Veiligheid, gezondheid en Milieu Aptil 2010. schrjft voor dat iedere faculteit een VGM plan moet hebben, wo bijv. De classificate Laboratoria, indien met GNO gewerkt wordt etc. Box 6,8 en 9 • Box 6 Third countries – Ook third countries approvals nodig indien in die landen data verzameld worden. ( ethics dumping) – Export vergunningen voor data (!) Material Transfer Agreements – Convention on Biodiversity • Box 8 en 9 Dual use and misuse – 8:Military en standards military ethics . – 9: malevolent, criminal/terrorist abuse. Box 10 Other • Box 10 Other Ethical issues • ??? • Cultural Heritage: – In Europa het verdrag van Valetta. Ethical experts at Leiden University Academic Integrity Committee Contact: Prof. I.M. Tieken-Boon van Ostade, i.m.tieken@hum.leidenuniv.nl 071-5272163 Visiting address: Rapenburg 70 2311 EZ Leiden Ethics Commission for Social Sciences and Humanities (SSH) The ethics committee SSH has been established by the deans of the Faculties of Archaeology, Campus The Hague, Humanities, Law and Social Sciences. Publication: Regulation Ethics Commission SSH Committee Secretary: Anne Christine de Haan LL.M. a.m.c.de.haan@cdh.leidenuniv.nl Ethics Review Board (ERB) Education and Child Studies Publication: Academic Integrity in the Institute of Education and Child Studies Committee Secretary: ms. Dr. D.J.G. Arnoldus d.j.g.arnoldus@fsw.leidenuniv.nl Ethical Committee Psychology (CEP) Application through: https://uleidenss.eu.qualtrics.com/SE/?SID=SV_4T5vINjCpgyb4Ve Contact person: mw. drs. M. Leemkuil ethiekpsychologie@fsw.leidenuniv.nl 071-5273725 Ethical experts at Leiden University continued Ethical Committee Medical Research (METC) (LUMC) Contact: cme@lumc.nl +31 (0)71- 52 63241 / 66963 Code integriteit wetenschappelijk onderzoek (LUMC) Publication: Scientific Integrity at the LUMC Information on Animal Welfare http://www.science.leidenuniv.nl/index.php/faculteit/diergebonden_onderzoek/onderzoekslijnen Information Management University Leiden Secretary: Mw. D.J. (Daphny) Uljé bvsecretariaat@bb.leidenuniv.nl +31 (0)71 527 3144 Contact person: Dhr.drs. M.C. (Marco) Gosselink MA m.gosselink@bb.leidenuniv.nl Ethics Appraisal Procedure – in 4 steps To assess and address the ethical dimension of activities funded under Horizon 2020 Step Activity Who? When? How? 1. Ethics self-assessment Applicant Application/submis sion phase Consideration of ethical issues of the proposal 2 Ethics Prescreening/Screening Ethics Experts Evaluation phase Review of application material 3 Ethics Assessment Ethics Experts Evaluation/ Grant preparation phase Review of application material 4 Ethics Check/Audit Ethics Experts Implementation phase Review of project deliverables/interview with applicants (for proposals involving hESC or raising serious ethical issues: severe intervention on humans) Ethics Appraisal procedure – its Life Cycle Call Deadline Submission Selfassessment Ranked Evaluation Prescreening Grant preparation Screening Project Closure GA Assessment Ethical Review Implementation Checks & Audits Audit Ethics Checks & Audits Ethical Appraisal You need to think about ethics as early as possible and throughout the research lifecycle!