Is There a Role for Analyses of Secondary Data in Assessing Drug
advertisement

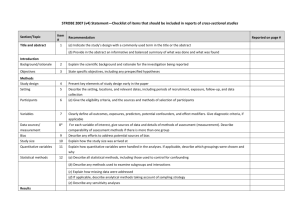
What Is the Role for Analyses of Administrative Data in Assessing Drug Safety in Post-Market Commitment (PMC) Studies? Cathy W. Critchlow, PhD Global Epidemiology, Amgen, Inc. September 29, 2006 Outline Why should we consider additional approaches (e.g., analyses of administrative data) to post-market commitment studies? What are situations where analyses of administrative data can be used to supplement, or even replace, clinical postmarket commitment studies? Study design issues Strengths and limitations of administrative data analyses as a component of post-market surveillance Post-Market Surveillance – Continuing Assessments of Safety or Efficacy Post-market surveillance Post-market commitment studies Registries or studies conducted to ‘complete’ pre-market assessments Routine surveillance Spontaneous reports • Unexpected or rare AEs Observational studies • Rate of expected/unexpected AEs • Relative rate of ‘hard’ endpoints ??? • Dynamic response to emerging issues, hypotheses Many examples Can analyses using automated databases help meet these needs? Need for Reevaluation of Post-Market Assessment Strategies Withdrawal of Cox-2 inhibitors after several years and several million patient exposures contributes to “perception of crisis that has compromised the credibility of FDA and the pharmaceutical industry”* Public’s loss of faith in the ability of industry to deliver safe and effective drugs† 13% think pharmaceutical companies are “generally honest and trustworthy” 60% not confident that drug companies will publicly disclose safety data in a timely manner *IOM Report: The Future of Drug Safety: Promoting and Protecting the Health of the Public †Harris Poll, 2004 Need for Reevaluation of Post-Market Assessments Strategies (2) Reliance on regulation alone to demonstrate longterm safety has not worked Unmet phase 4 commitments 114 (9.6%) of 1,191 open PMCs met* Confirmation of efficacy using hard endpoints in phase 4 commitment studies for drugs receiving “fast-track” approval based on surrogate measures FDA Critical Path Initiative Use of database registries and electronic medical record systems to compare outcomes among relevant patient groups in post-market drug evaluations * Federal Register 2005;70:8379-81. Crux of the Issue….. Post-Approval Drug Safety Typically, 500 - 3000 patients exposed to drug in phase 3 testing Drug effects detectable with an incidence ~1–6 per 1000 To quantify the risk of an event with incidence of 2 per 10,000/year (precision ±1 per 10,000) with 95% probability, need ~80,000 subjects followed for 1 year Difficult to conduct studies this large in a timely fashion Opportunities Consider additional or alternative strategies to demonstrate long-term drug safety or efficacy Analyses of administrative data* in post-market surveillance Collaboratively establish high standards for the conduct of observational data analyses conducted as part of post-market commitments Demonstrate safety and effectiveness of drugs in ‘real-world’ settings *Claims data (commercial insurance, Medicare, Medicaid), medical record data, national databases Qualities of the Ideal Database Comprehensive Inpatient & outpatient care; ER visits; lab & radiological tests; prescribed & OTC drugs; mental health care; alternative therapy Large, stable population Unique identifiers for linkage Regular, frequent updates Verifiable, reliable Capacity for chart review or patient interviews Confounder data Compliance But, few databases are ideal….(some are better than others) Problematic situations Illnesses that do not reliably come to medical attention Inpatient drug exposures Outcomes poorly defined by ICD-9 coding When necessary confounder data cannot be obtained Very long latency events Need to understand the limitations of any database Purpose for which database was created Data quality, validity, completeness Availability of confounder data Patient follow-up Access to source information Issues to Consider in the Design of PMCs What is the objective? Hypothesis generation (descriptive or exploratory) vs. confirmation? Evaluating expected vs. unexpected events Apriori specification of events of interest Timing of events of interest Short-term vs. long latency outcomes Most PMCs are Observational….. Issues of Study Validity Bias Selection bias Information bias Misclassification of covariates, exposure, outcome Data validity Confounding By disease severity, treatment indication, comorbid conditions Unmeasured covariates Time-dependent confounding Physician prescription patterns (‘channeling’) Dosing variability according to patient responsiveness What are appropriate comparator groups? Other Considerations…… Urgency of need for data Drug first in class or are other relevant data available? Risk vs. benefit profile Numbers of persons to be exposed Expected AE incidence rate Signal detection – what constitutes a safety signal? Implications for study design, sample size, comparators, interim analyses, scientific rigor required What are situations where analyses of administrative data can be used to supplement, or even replace, clinical PMC studies? PMC Scenario 1: Single-Arm Prospective, Observational Clinical Registry Characterize long-term safety profile of approved drug Incidence of various adverse events Drug utilization in the ‘real-world’ Special populations, e.g., children Effect of comorbid conditions Drug-drug interactions PMC Scenario 1: Single-Arm Prospective, Observational Registry Objective: Characterize drug safety post-approval Clinical Registry Hypothesis generating Pre-specified outcomes; unexpected events Modest sample size Will not observe rare events Effect measure Absolute incidence rate SIR (external comparator) Virtual (Database) Registry Hypothesis confirmation Post-hoc analyses of unexpected events Large sample size Effect measure Prospective data collection Difficult to assess long latency events Study rare events Absolute risk in select population Relative risk (internal comparator) Retrospective study design Potential for answers sooner PMC Scenario 1: Single-Arm Prospective, Observational Registry (2) Objective: Characterize drug safety post-drug approval Clinical Registry Data quality Outcome adjudication Covariate data can be obtained Regulatory definitions of AEs Sources of bias, confounding Potential selection, recall or information bias Differential loss-to-followup with respect to risk of outcomes? Virtual (Database) Registry Data quality Validity of algorithms assessing drug exposure, disease severity, outcomes Comparable ascertainment of data from exposed and comparator groups Data from all medical care providers Sources of bias, confounding Relevant covariate data available? Confounding by indication for treatment, comorbidities Stability of population When/What Could Analyses of Administrative Data Contribute to this PMC Scenario? Objective: Characterize drug safety post-drug approval When… Large sample size needed to assess rare events Events specified apriori Objective lab-driven diagnoses Confounder data available Denominator needed to calculate population incidence rates What….. Background incidence rates Attributable risk of events PMC Scenario 2: Controlled Studies Further Assessing Efficacy Obtain additional data regarding meaningful clinical endpoints Confirm estimates of efficacy of drugs receiving “fast-track” approval based on surrogate measures Head-to-head comparisons New vs. existing drug PMC Scenario 2: Controlled Studies Further Assessing Efficacy Objective: Compare incidence of clinical (efficacy) endpoints among exposed and unexposed groups Clinical Study Modest sample size Expense of large study of infrequent outcomes Effect measure Relative risk compared to placebo or standard of care Prospective data collection Potential ethical issues in randomized trial with placebo control and/or long follow-up Database Study Large sample size Study rare events Effect measure Relative risk More easily do ‘head-to-head’ comparisons Retrospective cohort study Potential for answers sooner PMC Scenario 2: Controlled Studies Further Assessing Efficacy (2) Objective: Compare incidence of clinical (efficacy) endpoints among exposed and unexposed groups Clinical Study Data quality Outcome adjudication Covariate data can be obtained Sources of bias, confounding Potential selection, recall or information bias Differential loss-tofollowup with respect to risk of outcome? Database Study Data quality Validity of algorithms assessing drug exposure, disease severity, outcomes Sources of bias, confounding Unmeasured confounders? Confounding by indication for treatment, comorbidities Stability of population When/What Could Analyses of Administrative Data Contribute to this PMC Scenario? Objective: Compare incidence of clinical (efficacy) endpoints among exposed and unexposed groups When… Large sample size needed to assess rare outcomes, longlatency outcomes Valid ascertainment of outcome Short-term effects Recall or interviewer bias could effect association What….. Timely validation of surrogate markers Head-to-head comparison of outcome incidence Opportunities - Revisited Potential role for analyses of administrative data in post-market surveillance Collaborative establishment of regulatory thresholds for the conduct, analysis and interpretation of observational data analyses conducted as part of post-market commitments Demonstrate safety and effectiveness of drugs in timely fashion Thank you!