Introduction to Geology 101
advertisement

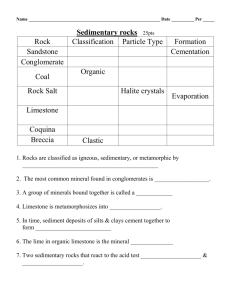
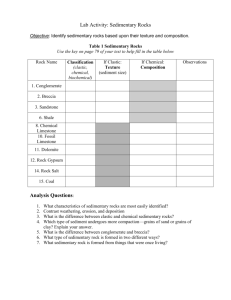
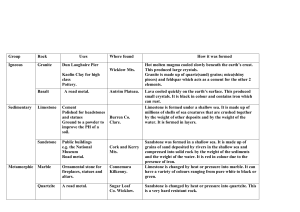
Chapter 5 – Sediment and Sedimentary Rocks Sedimentary rocks are composed of sediment. Sediment forms at or near earth’s surface through the processes of weathering, transportation, deposition, and lithification. Importance of sedimentary rocks include: Energy resources Petroleum Uranium Coal Sedimentary Resources Importance of sedimentary rocks include: Mineral resources Limestone: cement Rock salt: salt Rock gypsum: plaster Importance of sedimentary rocks include: Environment of deposition Oceans and seas Lakes and swamps River valleys and flood plains Deltas Sand dunes Every Rock Tells a Story Because sedimentary rocks form at earth’s surface, they tell the geologist what was happening on the surface when that rock was deposited. The rocks tell the story of Earth. For example . . . Limestone generally only grows in sea water. If a geologist finds limestone in Kansas, the geologist concludes that Kansas was once underwater. Sedimentary Rocks Sedimentary rocks are composed of sediment. Sediment can be either clastic (pieces) or chemical. Clastic sediment is pieces of preexisting rock like gravel, sand, silt and clay. Chemical sediment is chemicals dissolved in water. Sediment Sediment comes in two varieties. Sediment can be either clastic (pieces) or chemical. Clastic sediment is pieces of preexisting rock like gravel, sand, silt and clay. Chemical sediment is chemicals dissolved in water. Clastic Sediment Clastic sediment forms from the mechanical, physical and chemical breakdown of rock. The process of breaking rock down into smaller pieces aids further breakdown by chemical weathering. Chemical Sediment Chemical sediment forms by the chemical dissolution and alteration of rocks at or near earth’s surface. Chemical sediment is transported by stream waters to lakes and the oceans. Weathering Chapter 15 Mechanical Weathering Frost wedging Alternate freezing and thawing Temperate mountainous climates 9% expansion as water freezes Up to 80,000 pounds per square inch pressure Talus Slope Generally form a large pile of rock at the base of mountains called talus slope. Unloading As the weight of overlying rock is weathered and eroded away the rock expands and produces fractures (or joints) parallel to the topography. Exfoliation. Joints Vertical joints (cracks without displacement) form by expansion and contraction. Joints from Top with Striations Columnar joints with glacial striations. Devils Postpile National Monument. Thermal Expansion Joints Results from the daily cycle of temperature changes producing daily expansion and contraction. Most prominent in hot deserts. Biological Activity Plants and burrowing animals cause much joint expansion. Biological Activity The greatest mechanical weathering is now produced by catepillars. Biological Activity The greatest mechanical weathering is now produced by catepillars. Mechanical Weathering Enhances Chemical Weathering As mechanical weathering progresses, it enhances chemical weathering by increasing the surface area to volume ratio. Comparison of Surface Area to Volume Ratio The volume of a cube 2 cm on a side is: 2 x 2 x 2 = 8 cm3 The surface area of one side is 2 x 2 = 4 cm There are six sides, so the total surface area is 4 cm x 6 = 24 cm2 Comparison of Surface Area to Volume Ratio If the 2 cm cube is bisected by 3 joints how does this affect the surface area? Each face of the 1 cm cube has a surface area of 1 cm x 1 cm = 1 cm2. There are six sides to each cube so the surface area would be 6 x 1 cm2 = 6 cm2. But there are a total of 8 little cubes so the total surface area is now 6 cm2 x 8 = 48 cm2 Compare this to the previous surface area of 24 cm2. The surface area has doubled! Chemical Weathering The most important chemical causing chemical weathering is H2O-the universal solvent. However H2O in contact with CO2 gas in the atmosphere reacts to form carbonic acid. H2O + CO2 = H2CO3 with a pH of about 5.7; a weak acid. Dissolution Weathering When water precipitates (rains) on halite it will dissolve. NaCl + H2O = Na+ + Cl- + H2O Cave Formation (Karst Topography) When carbonic acid infiltrates into limestone or marble rock joints it begins to dissolve the calcite. CaCO3 + H2CO3 = Ca+ + HCO3- + CO2 Solid rock dissolves into calcium ions, bicarbonate ions and carbon dioxide gas. All dissolved in water which carries these products away leaving a cave. Cave roofs collapse to form cenotes or sinkholes. Cave When. Oregon Cave When. Hydrolysis of Granite 1 The most abundant rock in earth’s crust is granite. The most abundant mineral in granite is feldspar. Feldspar hydrolyses to kaolinite clay + bicarbonate + soluble silica. Hydrolysis of Granite 2 Feldspar + Carbonic acid = Kaolinite clay + Potassium + Sodium + Calcium + Soluble Silica (SiO2) Soluble silica is important because it becomes the silica cement that makes sand into a sandstone. White Sandstone sandstone. Hydrolysis of Granite 3 This weathering of feldspar in granite then releases the quartz grains to form sand that will become sandstone. The kaolinite clay becomes the clay mineral that makes shale. Shale is the most abundant sedimentary rock. Oxidation of Biotite Biotite mica in granite reacts with carbonic acid to make kaolinite clay and releases iron that can become the hematite cement that binds sand grains together to form sandstone. Red Sandstone sandstone. Factors that influence Weathering Minerals present Bowen’s Reaction Series Available liquid water Water is the most important weathering agent Tropics Antarctica Temperature As temperature rises rate of chemical reactions increases. Bowen’s Reaction Series Minerals present Bowen’s Reaction Series Available liquid water Water is the most important weathering agent Tropics Antarctica Temperature As temperature rises rate of chemical reactions increases. Liquid Water Minerals present Bowen’s Reaction Series Available liquid water Water is the most important weathering agent Tropics Antarctica Temperature As temperature rises rate of chemical reactions increases. Cold and Dry Weathering Minerals present Bowen’s Reaction Series Available liquid water Water is the most important weathering agent Tropics Antarctica Temperature As temperature rises rate of chemical reactions increases. Sedimentary Rock Pictures Gravel clasts. Chemical sedimentary rock. Clastic Sedimentary Rocks Clastic sediment, composed of rock and/or mineral fragments from pre-existing rocks. A convenient method to classify clastic material is by its clast (particle) size. Clastic Sedimentary Rock Classification Size Range (mm) Particle Name Rock Name > 256 mm Boulder Conglomerate 64-256 mm Cobble 4-64 mm Pebble 2-4 mm Granule (rounded clasts) Breccia (angular clasts) Quartz Sandstone 1/16 – 2 mm Sand Arkose Sandstone Graywacke Sandstone 1/256 – 1/16 mm Silt Siltstone < 1/256 mm Clay Claystone A rock that is about 1/3 silt and 2/3 clay and is fissile (laminated) Shale Sedimentary Environments - Shale Shale Most abundant sedimentary rock Deposition in quiet, non-turbulent water. Particles tend to form in thin lamellae. Deep sea environment or far away from high energy. Offshore and away from high-energy surf zone. Shale Pictures Shale Importance The dark colors of shale usually are caused by the deposition of small amounts of incompletely decayed organic material. Oil shale Methane shale Sedimentary Environments – Quartz Sandstone Quartz Sandstone: Quartz is highly resistant to weathering so it is what is left over when all the other common minerals have weathered away. Mature. Traveled the farthest. Sand dunes. Beach sand. Sedimentary Environments – Arkose Sandstone Arkose Sandstone: Contains roughly equal amounts of quartz and feldspar. Less mature than quartz sandstone and the presence of feldspar indicates deposition closer to source area (mountains). Much of the sand size material in the upper portions of the Santa Ana River near the the San Gabriel and San Bernardino Mountains is akose. Arkose Sandstone Picture Sedimentary Environments – Graywacke Sandstone Graywacke Sandstone: Usually dark colored rock composed of quartz, feldspar, rock fragments, mafic minerals and a matrix of fine clays and silts. Very immature. Common as turbidity currents fromed from submarine landslides associated with the edge of the continental shelf or trenches. Graywacke Sandstone Picture Sandstone textures Two important textures in sandstones. Rounding: the degree of rounding determines the transportation distance with more rounding indicating a longer transport distance. Sandstone Textures continued Sorting: the range of clast sizes. Usually determined by the means of transportation. Well sorted = most clasts are the same size. Poorly sorted = wide range of particle sizes. Sandstone Textures continued again Three transporting agents. Wind: very fine sorting. All sand grains nearly the same size. Water: intermediate sorting. Ice: very poor sorting. Clay size to boulders transported together. Sedimentary Environments – Conglomerate Conglomerates form in high energy environments. By floods Near mountains Steep terrains Usually poorly sorted. Nine foot boulder in back yard. Angular clasts indicate deposition very near source. Breccia. Sedimentary Environments – Conglomerate continued Rock fragments (clasts) may be used to determine the source area. Polka-Dot Granite of Joshua Tree National Park Conglomerate Picture Lithifying Agents Conglomerates and sandstones are lithified by cementation. Natural cements dissolved in groundwater precipitate minerals that end up transforming sediment into rock. Three cements. Quartz Hematite Calcite How could you distinguish these? Chemical Sedimentary Rocks Cemical sedimentary rocks form from the precipitation of chemicals from water. Salt encrusted iguana. Ready for serving? Oceans Salts Ocean Ions (Salts) Abundance Chlorine (Cl-) Sodium (Na+) Sulfate (SO42-) Magnesium (Mg2+) Bicarbonate (HCO3-) Calcium (Ca2+) Potassium (K+) Precipitation of these salts governed by chemical laws. Calcium carbonate is closest to the solubility limit despite low abundance. Ocean Salt Precipitation Order Calcite (CaCO3) - 50% water evaporated Gypsum (CaSO4 2H2O) - 80% water evaporated Halite (NaCl) - 90% water evaporated What would be the order of salt precipitation as the Mediterranean Sea evaporated? Limestones Little sea organisms have evolved protective armor against predators. What to make shells out of? calcium carbonate (calcite or aragonite-a polymorph) Many limestones are composed of the shells of sea organisms. Limestone Reef Reef Limestone Requirements Little sea organisms. What do they require? Warm water (>18° C 22-28 ° C optimum) - solubility Shallow water (bottom of food chain are plants that require sunlight) - <~200 ft water depth; 1% light left Little clastic sediment - covers plants Warm Water Locations . Great Barrier Reef . Great Barrier Reef . Limestone Types - Fossiliferous Limestone A limestone that contains fossils. . Limestone Types - Coquina A limestone that is composed of broken shell fragments. Anastasia Formation, Florida. Beach environment. . Limestone Types - Oolitic Limestone Composed of limestone and clay spheres. Typical of lagoons inbetween barrier island and mainland. . Limestone Types - Chalk White Cliffs of Dover. . Limestone Types - Chalk Single cell coccolith shell. . Limestone Types - Chalkboard Chalkboard slate. And you think you have it tough. . Limestone Types - Travertine Fly Geyser As calcium carbonate water cools at the surface calcite precipitates as layered travertine. . Limestone Types - Travertine - Havasupai Grand Canyon, Arizona. . Limestone Types - Travertine - Pamukkale Turkey. . Limestone Types - Dolomite Limestone rock reacts with magnesium in warm ocean waters. CaCO3 + Mg2+ = (Ca,Mg)CO3 + Ca2+ Since the magnesium ion is smaller than the calcium it replaces, the volume is decreases during dolomitization which increases porosity. A good reservoir for petroleum. Kuwait. . Limestone Types - Dolomite Qatar . Other Biochemical Sediment Chert - a rock composed of single cell shells of radiolaria. Typically deep sea sediement. . Other Biochemical Sediment Ribbon chert in central California. . Other Biochemical Sediment - Diatomite A rock formed of diatoms. . Other Biochemical Sediment - Diatomite Uses Fuller’s Earth: Removing sheep wool grease Swimming pool filter Added to toothpaste and baby food Dynamite: nitroglycerine stabilizer Insecticide Evaporites - Rock Salt Uses Food Need some salt in our diet Preservative - jerky Road salt (bad - sort of) Water softener (bad) Mined out areas for storage Evaporites - Camp Verde Salt Mine . Evaporites - Rock Gypsum Uses Wallboard Plaster Stucco Soil Amendment Breaks up Adobe soils Enviornment of Deposition Grand Canyon Muav Limestone Bright Angel Shale Tapeats Sandstone Using the Principle of Superposition, the Sandstone was deposited first (its at the bottom.) . Tapeats Sandstone Depositional environment was a beach . Bright Angel Shale Depositional environment was offshore of the beach in quiet water. . Muav Limestone Depositional environment was offshore away from shale deposition. . Rising Sea Level . Rising Sea Level - Glaciation? What can cause a rise in sea level? Glaciation - If we take water out of the oceans and place it on land as ice sea level can change by about 600 feet. At the peak of the last glacial cycle sea level was ~450 feet lower than today. If all the glaciers and ice caps melt, sea level would rise by ~150 feet. These cycles can take place in cycles of tens of thousands of years. Pleistocene Glaciation . Rising Sea Level - Plate Tectonics? What can cause a rise in sea level? When the rate of plate tectonics increases, the width of the mid-ocean ridges increases, displacing ocean water onto continental surfaces. Plate Tectonics . Plate Tectonics .