review powerpoint
advertisement

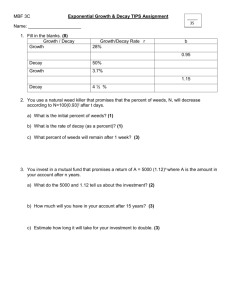
• List the two symbols that scientists use to represent a(n)… – Alpha particle? – Beta particle? α β Thorium-230 has a half life of 8000 years. How long will it take a 100 g sample of Th-230 to decay to 25 g? Find how many half-lives it takes to decay to 25 g. 100 50 25 2 half-lives. 2 x 8000 = 16,000 years Would Phosphorus-32 be stable? 15 protons, 17 neutrons YES! • When a nucleus undergoes alpha decay, what will happen to the mass? the atomic number? Mass decreases by 4 amu, Atomic number decreases by 2 • When a nucleus undergoes beta decay, what happens to the mass? the atomic number? Mass doesn’t change, Atomic number increases by 1 1. Identify the type of decay 2. How do you know? • Carbon-14 becomes nitrogen-14 • Rn (radon) decays to become Po (polonium) α • Lead (Pb) becomes Bismuth (Bi) • Polonium-214 becomes lead-210 β β α Write the equation for the radioactive decay of the following elements: • Uranium-234 (U, alpha decay) 234 U 92 4 He + 2 230 Th 90 • Bismuth-214 (Bi, beta decay) 214 Bi 83 0 e + -1 214 Po 84 An organism contained 200 grams of Carbon-14 when it died. After 17,190 years, only 25 grams remain. How long is Carbon-14’s half-life? Find how many half-lives have passed. 200 100 50 25 3 half-lives 17,190/3 = 5,730 years How many neutrons would a stable isotope of Tin (Sn) have? 58-75 Name the 2 forces working in the nucleus of an atom. Attractive Force: Strong Nuclear Force Repulsive Force: Electrostatic Force What is the difference between fusion and fission? Draw a picture showing the difference. Fusion: combining 2 nuclei, more energy released than fission, stars and hydrogen bomb Fission: splitting a nuclei, energy is released, nuclear powerplants and atomic bombs Write the products of the following fusion reactions: 4 + He 2 12 4 4 8 He 2 C + He 6 2 Be + Energy 4 16 8 O + Energy Write a radioactive decay equation that will produce/make magnesium-24 - Using alpha decay - Using beta decay 28 Si 14 24 Na 11 4 He + 2 0 e + -1 24 Mg 12 24 Mg 12 • Radium-222 (Ra, alpha decay) 222 Ra 88 4 He + 2 218 Rn 86 • Iodine-131 (beta decay) 131 I 53 0 - + e -1 131 Xe 54 Write a radioactive decay equation that will produce/make Silver-108: - Using alpha decay - Using beta decay 112 In 49 108 Pd 46 4 He + 2 0 e + -1 108 Ag 47 108 Ag 47