THE METRIC SYSTEM E-1
advertisement

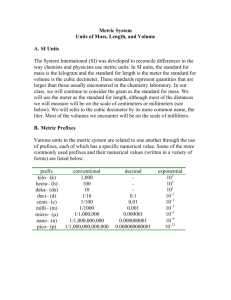
E-1 METRIC MEASUREMENTS Prerequisite to NGSS: HS: PS1-7 Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction. CC Energy and Matter. SEP Using Mathematical and Computational Thinking. SI System: All systems of measurement are arbitrary. That means anyone can devise a measuring system. The only requirements are that: 1) it has a basic unit, which can be subdivided to measure objects smaller than the unit; or, multiplied to measure objects larger than the unit, and 2) copies of the system unit are readily available for public use. Although the United States has not officially adopted the metric system as our measurement standard, you are probably aware that most packaged goods labels provide both British and metric equivalents. You may not know that most American auto-makers have changed automobile part specifications to metric. Why change? The first and most important reason is economic. We lose billions of dollars in trade each year because our goods are not metric standard. Second, the metric system is the standard system of measurement used in most every other country in the world. The metric system has been the standard system of measurement used by the worldwide scientific community since 1791, with good reason. The system is really a decimal system - that is, it uses the base 10; and the prefixes, indicating the power of 10, are cross-categorical - that is, they can be applied to more than one base unit. The following diagram illustrates this point: Larger unit to smaller unit, decimal moves to the right. Smaller unit to larger unit, decimal moves to the left. kilo103 (1000) hecto102 (100) deka101 (10) Base Units liter meter gram deci10-1 (0.1) centi10-2 (0.01) milli10-3 (0.001) Other useful prefixes are: mega- (106), micro- (10-6), and nano- (10-9). A meter is now defined as the length equal to the distance traveled by light in a vacuum in 1/299,792,458 seconds. The present standard for mass is kept at the International Bureau of Weights and Measures in France. Volume is a derived unit, a cubic decimeter. In 1960 the General Conference on Weights and Measures adopted SI units. This is a special version of the metric system which uses seven basic units. All other units, such as volume, density, and pressure, are derived from these. For example; 1 L = 1 dm3, and 1 mL = 1 cm3. QUANTITY length mass time temperature energy amount of substance electric current light intensity SI UNIT meter kilogram second Kelvin Joule mole ampere candela SYMBOL m kg s K J mol A cd 1 Chemists also use another non-SI unit of length, the angstrom (Å), which is 10-10 meter. Scientific Notation: Scientific notation is a way of expressing very small or very large numbers; e.g., 5,400,000,000 m. Any number can be expressed in scientific notation using the appropriate power of 10. When writing a number in scientific notation, move the decimal place so it is directly after the first nonzero digit. Then count the number of decimal places the decimal point was moved to determine the magnitude of the exponent (or power). If the decimal point was moved to the left, then the exponent is a positive number; if the decimal point was moved to the right, then the exponent is a negative number. Example 1: 5,400,000,000 m. The correct power is 109 since the decimal point is moved 9 places to the left. So, written in scientific notation it would be 5.4 x 109 m. Example 2: 0.00000989 L. The decimal point will move 6 places to the right, so the exponent is –6. The measurement would be 9.89 x 10-6 L. * Note that large numbers have positive exponents, and small (decimal) numbers have negative exponents. Conversion factors: Any equality can be written in fraction form and is then called a conversion factor. For example, 1 m = 100 cm. So, we can write these two fractions: 1m 100 cm or 100 cm 1m We write these fractions so that we can change from one unit of measurement to another. We can simply multiply by these fractions to and eliminate any units that are in both the numerator AND in the denominator. Example 1: 3456 cm2 = ________ m2 Rewrite your number and multiply by the fraction that would result in the correct units. We want to eliminate the cm and end with m, so we choose the first fraction. Remember that you must divide by any number in the denominator and multiply by any number in the numerator. 3456 cm2 1m 1m = 0.3456 m2. 100 cm 100 cm Example 2: 4.452 m2 = _______ cm2 4.452 m2 100 cm 100 cm = 44,520 cm2 1m 1m Notice that this time we use the other fraction, since we want to eliminate m and end with cm. Example 3: 3.53 m3 = _____ cm3 This time we have a cubed unit. 1 m3 does NOT equal 100 cm3! 1 m3 = 1m 1m 1m, so we need to use the conversion factor to eliminate the m3. 3.53 m3 ´ 100 cm 100 cm 100 cm = 3,530,000 cm3 or 3.53x106 cm3 ´ ´ 1m 1m 1m 2 E-1 PRACTICE PROBLEMS Metric Prefixes 1. Using metric prefixes, list the names and abbreviations for 6 different units of length. 2. Using metric prefixes, list the names and abbreviations 6 different units of mass. 3. Using metric prefixes and/or superscripts, list 6 the names and abbreviations different units of volume. 4. Convert each of the following quantities to the desired unit: a. 14.06 meter = ____________ kilometer b. 38 liter = ____________ milliliter c. 6060 dekaliter = ____________ deciliter d. 18 hectogram = ____________ kilogram e. 400 centimeter = ____________ millimeter f. 4.2 kilogram = ____________ gram g. 0.843 gram = ____________ centigram h. 8060 millimeter = ____________ meter i. 600. milliliter = ____________ liter j. 77 kilometer = ____________ millimeter 5. Convert each of the following quantities to the desired unit: a. 32.6 cm = ____________ mm b. 468 mg = ____________ cg c. 0.038 m = ____________ cm d. 1400 mL = ____________ kL e. 1.8 dL = ____________ mL f. 8.60 kg = ____________ hg g. 34 hg = ____________ mg h. 5.06 L = ____________ daL i. 13 mL = ____________ cL j. 57 cg H2O = ____________ g H2O Scientific Notation 6. Write the following measurement in scientific notation. a. 45,500,000 mL b. 0.00000452 cm c. 834,000,000,000,000 m d. 0.0034 mol e. 9,000,000,000 molecules f. 0.000000000023 L 7. Change the measurements to standard form. a. 4.34 x 10-7 m b. 9.0 x 105 L c. 5 x 104 K d. 3.787 x 10-8 cm e. 9.1 x 10-4 km f. 3.34 x 105 g 3 Conversion Factors 8. 9. Complete the following conversions using the conversion factors. a. 4 m2 = ____________ cm2 b. 800,000 cm2 = ____________ m2 c. 5 km2 = ____________ m2 d. 140 m2 = ____________ mm2 e. 54.6 m2 = ____________ km2 f. 0.006 m3 = ____________ mm3 g. 4864 cm3 = ____________ m3 Complete the following conversions using the conversion factors. a. 235 cm3 = ____________ mL b. 0.457 cm3 = ____________ L c. 0.215 L = ____________ cm3 d. 12,434 dm3 = ____________ mL e. 608 mL = ____________ dm3 4