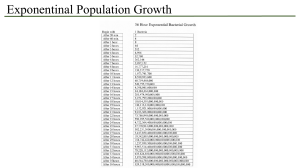
Thrips palmi contingency plan for cucumbers and other salad crops
advertisement

CENTRAL SCIENCE LABORATORY DEPARTMENT FOR ENVIRONMENT FOOD & RURAL AFFAIRS Contingency planning for outbreaks of Thrips palmi on protected cucumbers Compiled by Lynne Matthews, Ray Cannon, Keith Walters and Dominic Eyre Central Science Laboratory, Sand Hutton, York, YO41 1LZ With additional contributions from Rob Jacobson and Derek Hargreaves Cucumber Growers Association 23rd March 2005 CONTENTS SUMMARY 1. Introduction 2. Prophylactic use of foliage and flower inhabiting natural enemies 2.1 Species currently available in the UK 2.2 Species available overseas (not yet registered in the UK) 3. Prophylactic use of generalist natural enemies on the ground 3.1 UK products 4. Remedial use of invertebrate natural enemies 4.1 UK products 4.2 Overseas products 5. Remedial use of entomopathogenic fungi 5.1 UK products 6. Chemical control 6.1 UK products 6.2 OVERSEAS PRODUCTS 7. PHYSICAL CONTROL 8. RECOMMENDATIONS (HIGH PRIORITY IN BOLD) 9. REFERENCES APPENDIX 1 - Cucumber Grower Association Proposed Strategy for the Management of Thrips palmi in Cucumber Crops APPENDIX 2 - A modular approach to integrated control of Thrips palmi Karny: Concept note 2 Summary Thrips palmi is not established anywhere in Europe, and although it has been successfully eradicated from an ornamental crop in the UK, there are a dearth of effective measures which could be utilised in the event of an outbreak in an edible crop. Lessons learnt in an ornamental situation would not necessarily apply to an edible crop, when Integrated Pest Management (IPM) is practised. T. palmi is notoriously difficult to control by chemical means alone and effective IPM measures need to be developed which can be utilised in a UK situation. T. palmi occurs out-of-doors in many tropical and sub-tropical countries, and a wide range of potential natural enemies have been identified, in some cases providing highly effective control, suppressing the pest to low population levels. In the UK, the main economic threat is to protected edible crops such as cucumbers, aubergines and sweet peppers, where current pest management relies heavily on biological control. Although there have not been any outbreaks of T. palmi on cucumbers in the EU, there was a finding of a single adult on a cucumber nursery in the Beverley area, Yorkshire, in 1999. This, together with a similar ‘trace’ find on cucumbers in the Netherlands, prompted concern among UK growers and led to the Cucumber Growers Association (CGA) approaching Plant Health Division (PHD) with a proposed strategy for T. palmi. This then led on to meetings with CSL, which resulted in the present document and recommendations for work in support of an improved contingency plan for this species on cucumber. This modular strategic approach to T. palmi control is reflected in the recommendations. In equivalent cucumber growing situations where the pest is established – such as Japan – biological control systems are in general less well-developed than in Western Europe, and furthermore, utilise native biological control agents (BCAs) which are both unavailable and unregistered in the UK. In other words, they would be classified as non-native organisms in the UK, thus requiring extensive testing and regulatory approval (via the Advisory Committee on Releases to the Environment, ACRE) before they could be released. Thrips palmi is a highly cryptic pest, with a reputation for rapidly developing resistance to a wide range of chemical insecticides. Although a number of effective products are potentially available for T. palmi control, many of these are not available in the UK. Of those that are registered, some are either not approved for use in edible crop situations or would have the effect of disrupting existing biological control measures for other pests. One notable exception is spinosad, which is registered in cucumbers and which is reportedly compatible with beneficials. The aim of this work has been to review the range of existing control measures for T. palmi, worldwide, and to select those with the potential for further investigation. Recommendations are provided for the assessment of 3 both biological and chemical controls methods, categorised according to whether or not they are currently available – i.e. registered – in the UK. Assessments and selection of appropriate controls will have to be carried out under quarantine conditions, which put a constraint on the level of experimentation that can be carried out. Further work will also be necessary to adapt the most efficacious methods selected, into IPM programmes, and to consider how these can be best adapted to achieving the goal of eradication of the pest, whilst minimising the impact on the Industry. 4 1. Introduction Thrips palmi Karny (Thysanoptera: Thripidae), the melon thrips, is widely distributed in tropical and sub-tropical regions, including Southeast Asia, the Pacific Islands, the Caribbean Islands and South America (Murai, 2001). In 1978, T. palmi was found in Japan for the first time and it rapidly became the most serious pest of aubergine, cucumber and sweet pepper both in greenhouses and out-of-doors. It has also invaded southern Florida and Australia and is a serious threat to these protected crops in Europe, where it is listed as a IAI pest in EC Plant Health Legislation (Directive 2000/29/EC). To date, there have been 26 outbreaks of T. palmi in the EU - 25 in the Netherlands and one in the UK - all of which were successfully eradicated by a combination of crop destruction and intensive insecticide applications. In the Netherlands, outbreaks occurred on Cactaceae and Ficus benjamina between 1988 and 1994. The UK outbreak was detected in April 2000 on an all-yearround (AYR) chrysanthemum crop on the south coast, although the outbreak was well advanced and probably long established at this time. T. palmi was declared eradicated in July 2001 after freedom from the pest occurred over two complete cropping cycles (Cannon et al., in prep.). Although many lessons on how to control T. palmi were learnt during this successful eradication of T. palmi on an ornamental crop, they do not necessarily apply to an outbreak on cucumbers. For example, biological control was not being practised on the infested chrysanthemum crop, and the availability of insecticides is much greater for ornamentals than for edible crops. In addition to these high-profile outbreaks in the Netherlands and the UK, which were on ornamentals, there were two ‘trace’ finds on cucumber crops (one in the UK and the other one in the Netherlands) which prompted great concern among growers, and were the spur to the formulation of a proposed strategy for T. palmi on cucumber crops and to the formal approaches to the Plant Health Division (PHD) by the Cucumber Growers Association (CGA). The UK find was a single adult female T. palmi, which was detected on cucumber plants at a large cucumber nursery in the Beverley area, Yorkshire in 1999. Despite extensive re-sampling, no further specimens were detected; thrips control on this site was to a high standard. Fortunately, the find occurred towards the end of the cropping season, and a thorough clean-up programme was implemented during the crop break. The following season, the crop received an intensive biocontrol programme against the western flower thrips, Frankliniella occidentalis (WFT), which might also have prevented further establishment of T. palmi. In 1998, the year before this ‘trace’ find, the Netherlands reported to the European Commission, that a single T. palmi larva had been found on cucumber at a nursery in the NoordBrabant region. Thus, cucumber (and other edible cops such as aubergine and peppers) are at a very real risk from this invasive thrips. T. palmi remains a major pest of several greenhouse crops in Japan, including aubergine, sweet pepper and watermelon (Yano, 2004). However, the highest reproductive rates and the maximum intrinsic rate of natural increase of T. palmi populations occurs on cucumbers (Murai, 2001), so it is 5 this crop which is most at risk. T. palmi tends to live and feed on the leaves of cucumber plants; the leaf edges curl downward after heavy thrips feeding and serious damage often occurs during the early crop stages. Thrips feed by bursting the cells of the host plants and sucking up the exposed cell sap, resulting in discoloration of plant tissues leading to scarring and distortion of plant and produce (Kirk, 1997). Given its tendency to feed on the foliage, T. palmi is often reported to be less damaging to cucumber fruit than WFT (Rosenheim et al. 1990). Nevertheless, fruits may also be damaged; scars, deformities, and abortion are reported. Densities from one to ten T. palmi per cucumber leaf have been considered to be the threshold for economic damage in some Japanese studies (Capinera, 2000). However, studies in Hawaii suggested a damage threshold of 94 thrips per leaf early in plant growth (Welter et al. 1990). Although there have been no reported outbreaks of T. palmi on cucumbers in the EU, the singleton finds, detailed above, illustrate that there is a pathway for the introduction of T. palmi to UK cucumber crops, although it is not clear how this occurred (Cannon et al., in prep.). T. palmi is a notoriously difficult pest to control, with many studies demonstrating that the use of chemicals alone will not be effective (Kawai, 1990; 2001; Rosenheim et al., 1990; Young and Zhang, 1998). Equally, successful biological control of T. palmi is not achievable at present (Website 2), partly because biological control using arthropods is not well developed in countries such as Japan (Wada, 1999; Website 6), where the pest is present . An integrated pest management (IPM) system has been suggested as the way forward for the control of T. palmi (Kawai and Kitamura, 1987 in Walker, 1994) and this is likely to be the best option for eradicating outbreaks of T. palmi in edible UK crops. IPM programmes combine a range of control measures, including: the use of biological agents (BCAs); physical methods such as trapping, and the use of plastic mulches and other barriers; cultural measures such as rouging and manipulation of the crop environment; as well as carefully targeted pesticides applied at critical times (Lenteren, 1995; Jacobson, 1997; Loomans and Vierbergen, 1999). The aims of this report are therefore to: i) identify and review the availability and efficacy of a range of biological, chemical and physical products (or treatment options) for use against T. palmi on cucumbers; comparing what is currently registered in the UK with what is available elsewhere, worldwide; and ii) develop an effective IPM treatment schedule and eradication strategy for T. palmi, in the event of an outbreak on cucumbers in the UK. Components of the strategy might also be applicable to other protected salad crops in the UK. 6 2. Prophylactic use of foliage and flower inhabiting natural enemies 2.1 Species currently available in the UK The predatory mite, Amblyseius cucumeris (Mesostigmata: Phytoseiidae), is currently used to control F. occidentalis in UK cucumber crops; it is recommended that culture sachets be put on plants as early as possible and replaced when they stop producing predators (approximately every 8 weeks) (Jacobson et al., 2001b). A. cucumeris is also effective against T. palmi in aubergines, strawberries and cucumbers under greenhouse conditions. One mite can consume, on average, 65.3 first instar larvae of T. palmi and 18.7 second instars during its life cycle, and females consume more prey as the thrips density increases (Cuellar et al., 2002). A. cucumeris was registered for use as a BCA (‘Cucumeris’ ®) in Japan in April, 1998 (Wada, 1999). It is produced by ‘Techno Green Company’, a subsidiary of ‘Nippon Kayaku’ and distributed through the ‘Tomen Corporation’ (Website 3). The recommended dose is 100 mites per cucumber plant sprinkled at the base of each plant at the beginning of insect occurrence; at least 2-3 consecutive weekly releases are recommended. A. cucumeris is also used to control T. palmi on pepper; 100 adults per plant, released three times at 1-week intervals (starting 3 days after planting), reduced a population of T. palmi to between one third to one fifth of its original size, for 6 weeks (Kurogi et al.,1997). A. cucumeris must be applied to each cucumber plant, because a lack of overlapping foliage early in the season hinders the dispersal of the mites within the crop (Website 3). Amblyseius sachets are not used in Japan because the authorities do not permit the importation of Tyrophagus mites, which are incorporated into the sachets as a prey source for the Amblyseius; Tyrophagus mites may cause slight damage to some crops, especially when growing conditions are humid and they are present in large numbers (Website 5). In conjunction with this, there has been no investment in the machinery required to produce the culture packs (Rob Jacobson, Cucumber Growers Association, pers. comm.), but the Amblyseius sachet system would be the standard method of introducing A. cucumeris to an outbreak of T. palmi on cucumbers in the UK. A rate of 1000 A. cucumeris per plant is used to control F. occidentalis and it is suggested that at least this amount would be required to control T. palmi (Rob Jacobson, pers. comm.). Amblyseius spp. are relatively inexpensive in comparison with predatory Hemiptera such as Orius spp. (see section 4) and populations can increase more rapidly. However, as with some Orius spp., A. cucumeris is limited in its early and late season uses by a tendency to enter diapause in response to short daylengths, a state where the mites continue feeding but do not reproduce (Chambers and Long, 1991), although some biocontrol companies sell strains which are not susceptible to diapause, e.g. ‘Thripex’ by ‘Koppert’ (Website 5). Koppert are developing a new BCA: the predatory mite Typhlodromips swirskii. In trials it has given excellent thrips control in sweet pepper and 7 cucumber (Website 5), although as a non-native animal it will require a licence for release in the UK (David Flory, Koppert, pers. comm.). Phytoseius spp. (Mesostigmata: Phytoseiidae) mites are also known to prey on thrips, including T. palmi; they are recorded feeding readily on first instar larvae of T. palmi in Thailand (Hirose et al., 1993), but the only product registered for use in the UK, P. persimilis, is almost completely dependent on spider mites and is marketed for the control of spider mites only. Some generalist predators, available for use in the UK, may also have some potential for use in a T. palmi eradication programme: e.g. the bug, Anthocoris nemoralis (Hemiptera: Anthocoridae), and larvae of the lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae). A. nemoralis is primarily sold for the control of pear psyllid in orchards, but will feed on a range of small insects, including thrips and mites. Diapause restricts its use to between March and October in the UK (Clare Sampson, Biological Crop Protection, pers. comm.). In trials, C. carnea had a medium consumption of 14.4 T. palmi nymphs in 24 hours (Alvarez et al., 2002). 2.2 Species available overseas (not yet registered in the UK) Several non-indigenous mites are recorded feeding on T. palmi, including some exotic Amblyseius spp. and Typhlodromalus aripo (Mesostigmata: Phytoseiidae). Adult female A. mckenziei and A. okinawanus have been detected preying on T. palmi on cucumbers in Japan; they prefer first instar T. palmi to second instars and adult thrips (Kajita, 1986). In other tests, adult A. maai and A. asetus consumed 80.2 and 90.2 T. palmi larvae, respectively, and it was concluded that the latter had the potential to be used for the biological control of thrips pests (ChyiChen and WenHua, 2001). A. longispinosus (Hirose, 1989b) and A. tsugawai (CABI, 2004) have been recorded in Japan. CABI (2004) also list A. bakeri amongst the natural enemies of T. palmi. T. aripo can consume 72.9 first instar larvae of T. palmi and 21.6 second instars during its life cycle and females respond positively to higher densities of thrips (Cuellar et al., 2002). Recently, a new biocontrol product has been developed containing A. montdorensis, which has higher rates of prey consumption and a higher reproductive potential than A. cucumeris when used in its optimum temperature range of 20-30°C. (Richard GreatRex, Syngenta Bioline, pers. comm.). In trials, it has been found predominantly on leaves or fruits and not in flowers. This apparent preference for leaves makes the species of interest for the control of T. palmi, although so far performance against this pest has not been tested (Cox et al., unpublished CSL document). A range of other natural enemies of T. palmi have been reported; CABI (2004) list 23 different species, including: Megaphragma (Hymenoptera: Trichogrammatidae), a parasitoid of eggs in Thailand; Carayonocoris indicus (Hemiptera: Anthocoridae), attacking larvae and adults in Japan, and Propylea japonica (Coleoptera: Coccinellidae), attacking larvae in Japan. However, the only other natural enemy to be sold commercially appears to be the predatory thrips, Franklinothrips vespiformis (Thysanoptera: Thripidae). F. 8 vespiformis provides effective control of T. palmi in greenhouses in Okinawa Prefecture, Japan (Yano, 2003), where it is registered for use in protected aubergines and cucumbers (Yano, 2004). It can effectively locate and feed on the eggs of T. palmi embedded within plant tissues (Loomans and Heijboer, 1999). Campylomma chinensis (Hemiptera: Miridae) is also recorded as a predator of T. palmi in Japan (Hirose et al., 1999) and Taiwan (Parker et al., 1995). Final instar larvae and adults of C. chinensis consume 20 and 27-29 T. palmi per day, respectively (Parker et al., 1995), whilst the related species, C. livida has been identified as an important natural enemy in Thailand and the Philippines (Searca, 1991). Adult C. livida attack second instar larvae of T. palmi (Hirose et al., 1993). Other predators of T. palmi reported in the literature are the stilt bug, Yemma exilis (Heteroptera: Berytidae), which has been observed feeding on adults and larvae of T. palmi on aubergine in a plastic greenhouse. In experiments, the mean number of T. palmi consumed by a female bug was 4-7 for the first 5 days (Kohno and Hirose, 1997) and in Trinidad, the beetle, Coleomegilla maculata (Coleoptera: Coccinellidae), has been found preying on T. palmi, although it is not indigenous to the country (Cooper, 1990). The literature also reports two larger-scale surveys (in Thailand and Vietnam) carried out to identify potential BCAs for T. palmi. Of eight natural enemies found in Thailand, the eulophid larval parasitoid, Ceranisus menes (Hymenoptera: Eulophidae), was found to be the most effective; 40-60% of larvae were parasitized in domestic gardens that were not sprayed with insecticides (Hirose et al., 1993). In contrast, Castineiras et al. (1996a) found parasitism by C. menes to range from c. 24-29% at 25-29ºC. The rate of parasitism decreased to 11.5% at 21ºC. Ceranisus spp. are also effective against T. palmi in Japan (Hirose, 1989a). The larval predator Bilia sp. (Hemiptera: Anthocoridae) was the second most effective natural enemy in the survey in Thailand; both adults and nymphs attack T. palmi larvae (Hirose et al., 1993). Another anthocorid, Wollastoniella parvicuneis, also preys on T. palmi in aubergine crops in northern Thailand (Yasunaga, 1995). Cage trials in Japan in winter greenhouses showed that W. rotunda successfully developed, reproduced and suppressed T. palmi populations, and it was concluded that it had the potential to be an effective control agent for T. palmi on aubergines (Nakashima et al., 2004). However, in an IPM programme, interspecific competition may occur between anthocorid species. Finally, a survey of indigenous natural enemies of T. palmi in Gia Lam Hanoi (Institute for Fruit and Vegetables Research) in Vietnam found 14 species of indigenous natural enemies – 13 predators and one parasitoid. Those not mentioned previously are Xylocoris sp., Lyctocoris beneficus and Amphiareus obscuriceps (Hemiptera: Anthocoridae); Isometopus japonicus and Orthotylus sp. (Hemiptera: Miridae); Haplothrips sp. (Thysanoptera: Phlaeothripidae); Aeolothrips sp. (Thysanoptera: Aeolothripidae); Scolothrips sexmaculatus (Thysanoptera: Thripidae); Menochilus sexmaculatus, Micrapis discolor and Oenopia sauzati (Coleoptera: Coccinellidae) (Try and Hung, 2003). 9 3. Prophylactic use of generalist natural enemies on the ground 3.1 UK products The soil predatory mite Hypoaspis miles (Mesostigmata: Laelapidae), commercially produced for fungus gnat control in the UK, will feed on thrips that drop to the ground to pupate (Brodsgaard et al., 1996; Gillespie and Quiring, 1992). It is a useful predator of low-level pest populations, since adults can live for up to 70 days in the absence of food, providing they have fed previously. It is also very mobile and is tolerant of many insecticides applied to the foliage of crops (Website 4), probably as a result of spatial separation from these chemicals. Up to 30% reduction in damage caused by F. occidentalis is seen where H. miles is used (Richard GreatRex, Syngenta, pers. comm.). Recommended release rates vary from 100 per m 2 (‘preventative’) to 500 per m2 (‘curative heavy’) (Website 5). Atheta coriaria (Coleoptera: Staphylinidae) is a soil-dwelling rove beetle native to the UK. Laboratory trials in Canada have shown that it is capable of consuming pupae and late larvae of F. occidentalis and it may have some potential for the control of T. palmi (Richard GreatRex, Syngenta Bioline, pers. comm.). However, hydroponic cucumber crops do not provide a favourable environment for soil-dwelling predators, due to the plastic floor covering (Derek Hargreaves, Cucumber Growers Association, pers. comm.). A mesh covering might improve the environment, but further research on the efficacy of H. miles and A. coriaria against T. palmi would be required before such expense could be justified. 4. Remedial use of invertebrate natural enemies 4.1 UK products The predatory bugs, Orius majusculus and O. laevigatus (Hemiptera: Anthocoridae), are used to control thrips in the UK; O. laevigatus is well adapted to living in confined spaces, which could increase its chances of coming into contact with T. palmi (Chambers et al., 1993). However, the efficacy of these species against T. palmi is unknown and they enter diapause in response to short daylengths, limiting their potential for use in UK crops. Critical day length varies between species; for O. majusculus it is between 14 and 16 hrs, limiting establishment in UK crops between September and April (Jacobson, 2003). O. laevigatus requires a minimum daylength of 10-11 hrs. Supplementary lighting has been used to establish localised populations of O. majusculus from early March (Jacobson, 1993), and releasing adults only might bring forward the establishment of Orius spp. by 3-4 weeks (Jacobson, 1993), since there is evidence that it is the final nymphal stage that is susceptible to diapause (Ruberson et al., 1991). An alternative approach might be to use non-diapausing subtropical strains, such as those that occur for O. laevigatus (Shipp and Ramakers, 2000). However, it is likely that non-native strains would need to be licensed before being released in the UK and there are additional, complicating factors that 10 limit the establishment of Orius spp. in cucumber crops. For example, large populations of thrips are required to ensure settlement of O. laevigatus due to the lack of pollen in cucumber crops (Chambers et al., 1993). In the absence of suitable (or sufficient) food, or if released in hot conditions, adult Orius sp. will fly and may leave the crop area. To overcome this problem, ‘Syngenta Bioline’ have been trailing a product containing only first and second instar Orius nymphs (Richard GreatRex, Syngenta, pers. comm.), but this again raises the problem of diapause. In addition, the most common egg-laying sites for O. majusculus are within the youngest 50 cm of growth. During routine crop maintenance, lateral shoots are removed, which could result in the loss of 30% unhatched eggs of O. majusculus. Furthermore, some eggs laid in the short-lived flowers may not complete development (Jacobson, 1995). The practice of re-planting cucumber crops in mid-summer to maintain fruit quality also remains a potential obstacle to the establishment of Orius spp. (HDC, 2000a; Jacobson, 2003) due to their relatively long life-cycle: 3-4 weeks under spring and summer conditions in cucumber and pepper crops (Jacobson, 2003). Foliar applications of entomopathogenic nematodes, such as Steinernema feltiae (Steinernematidae), could be used to re-address the balance between T. palmi and its natural enemies in pest ‘hot-spots’, i.e. where BCAs are not adequately controlling the thrips. At CSL, applications of S. feltiae suspensions (5000 infective juveniles per ml) containing the adjuvant ‘Agral’ (0.02% v/v) achieved significantly greater mortality of T. palmi second instar larvae than control treatments; similar mean levels of mortality (30-41%) were achieved on three hosts – chrysanthemum, cucumber and capsicum. (CSL, 2003). To maximize nematode efficacy, applications should be made to the foliage at 20C and followed by an 8hr period of high humidity (>90%) (CSL, 2003). There are however, concerns as to whether elevated relative humidity could be maintained under commercial conditions (see also comments for entomopahogenic fungi, below). S. feltiae is compatible with 24hr old residues of the following insecticides recommended in the PHSI Eradication and Containment handbook for T. palmi: abamectin (‘Dynamec’), deltamethrin (‘Decis’) and nicotine (‘XL-All Nicotine 95%’) (Justine Head, CSL, pers. comm.). 4.2 Overseas products In Japan, five indigenous Orius species, O. sauteri, O. minutus, O. strigicollis, O. nagaii and O. tantillus, are considered to be major natural enemies of thrips in the field (Yasunaga, 1997); of these O. sauteri, O. strigicollis and O. tantillus all prey on T. palmi (Yano, 1999). Orius spp. are capable of preying on adult thrips and the number of 1st and 2nd instar larvae consumed by 2nd instar nymphs of Orius spp. does not differ greatly (Kajita, 1986). O. sauteri is considered to be the most important natural enemy of T. palmi in sweet pepper and aubergine fields (Hirose, 1990; Kajita, 1985), and suppressed the incidence of T. palmi on aubergine in greenhouse trials (Kawai, 1995) and in a screen-house (Nagai et al., 1988b). It will feed on the 11 adults, larvae and eggs of T. palmi (Paik et al., 2003). O. sauteri was registered as a biopesticide in Japan in 1998. On pepper, O. sauteri (5 adults per plant) released twice at 1-week intervals, starting 3 days after planting, reduced the T. palmi population to less than or equal to 1/5 of the original size for 2 months after planting (Kurogi et al., 1997). However, the induction of reproductive diapause limits the efficacy of Orius spp. The low reproduction rates and predation rates of O. sauteri under low temperature conditions mean that this species is ineffective in winter (Nagai and Yano, 1999, 2000). O. strigicollis is preferred to O. sauteri for commercial use because of its lower diapause incidence and the ease for mass production (Shimizu and Kawasaki, 2001; Yano, 2004); it was registered as a biopesticide in Japan in 2001 and is widely used for controlling T. palmi on aubergines and sweet peppers in Kochi Prefecture (Yano, 2003). A single O. strigicollis usually eats more than 200 thrips during its lifetime (including immature and mature stages, and assuming a stable temperature of 25ºC) (Wang, 1994). Releases of 100-150, two-three day-old nymphs of O. strigicollis per aubergine per week in an open field in Taiwan also decreased the number of T. palmi from 50 per leaf to 3 per leaf within 5 weeks (Wang et al., 2001). O. minutus shows almost the same reproductive diapause as O. sauteri (Kohno, 1997, 1998; Ito and Nakata, 1998). O. tantillus and O. nagai are not considered as suitable species to be used for biological control of pests of greenhouse vegetables (Yasunaga, 1997). Sakimura et al. (1986) refer to a sixth species, O. similis, which has been detected preying on T. palmi in China; second instars are said to consume about 10 T. palmi per day (Wei et al., 1984). In India, Kumar and Ananthakrishnan (1984) studied Orius maxidentex in the laboratory and in a field near Madras, where it preyed on T. palmi on the young foliage of sesame. After the crop was harvested, it was abundant on the weed Croton sparsiflorus, preying on T. palmi until prey populations died out in September. O. insidiosus is recorded as a predator of T. palmi in Hawaii (Mau et al., 1989) and is reportedly abundant on aubergine in Guadeloupe (Etienne et al., 1990). If Orius spp. are to be used in eradication programmes for T. palmi further work is required to assess their compatibility with insecticides. Insect growth regulators, such as ‘Nemolt’ (teflubenzuron) tend to disrupt the development of Orius, as does ‘Vydate’ (oxamyl) (Website 4). However, Nagai (1993) reports that O. sauteri is tolerant to the insecticides pyriproxyfen and buprofezin, both of which are growth regulators. 5. Remedial use of entomopathogenic fungi 5.1 UK products Lecanicillium mucarium (Hyphomycetes) (formerly Verticillium lecanii) has been used to control T. palmi in greenhouses in Japan; Saito (1992) applied four sprays per week in May-June against T. palmi and Bemisia tabaci on melons and maintained populations of both pests at low levels. CSL (2003) showed that L. mucarium was most effective against T. palmi at 25C and that efficacy against the adult stage was significantly higher when used with the 12 adjuvant ‘Agral’. However, the relative humidity within a cucumber crop may be insufficient for the use of L. mucarium (Derek Hargreaves, pers. comm.). HDC (2000a) tested L. mucarium in combination with the dextrose starch adjuvant ‘Hugtite’, but the benefits were uncertain and the adjuvant could encourage the growth of Cladosporium spp. on the leaves. 5.2 Overseas products UK trials have suggested that the entomopathogenic fungus, Beauveria bassiana (Hyphomycetes), is effective at a lower relative humidity than V. lecanii (HDC, 2000a) and can be used to augment releases of A. cucumeris in the preventative management of F. occidentalis; glasshouse populations of F. occidentalis were reduced by 65-87% with three consecutive high volume sprays or low volume mist applications of ‘Naturalis-L’ or ‘BotaniGard WP’, applied at 6-day intervals (Jacobson et al., 2001a). A. cucumeris on the leaves were unaffected by high volume sprays of B. bassiana directed at F. occidentalis and there was no indication that the applications were harmful to the predators or their prey in opened culture packs that were on the plants when the sprays were applied (HDC, 2000a). This suggests that B. bassiana could be incorporated into an IPM programme for thrips pests. However, in trials specifically with T. palmi, the results have been less encouraging. The potential of B. bassiana (strains BbH and BbHa) and Paecilomyces fumosoroseus (strain 97) for the control of T. palmi was studied on green beans in greenhouse experiments. P. fumosoroseus produced only 0.20% mortality; 24% mortality of thrips larvae treated with B. bassiana (strain BbHa) was achieved, but the infection only developed when leaves with larvae were incubated after being sprayed with the pathogen (Castineiras et al., 1996b). CSL (2003) also had poor results when using B. bassiana against T. palmi; it was found to be less effective against the larval stage of T. palmi than S. feltiae or V. lecanii (CSL, 2003). B. bassiana is also known to naturally infect the minute pirate bug O. insidiosus (Frantz and Mellinger, 1998), which may prevent it being used in IPM programmes that utilise this predator. The best results with B. bassiana are obtained when the spray directly hits the target. Therefore, good spray cover is essential and the spray techniques used in commercial UK cucumber crops may need to be improved before B. bassiana can be used successfully (HDC, 2000a). Alternatively, B. bassiana may be used to control thrips pupae; a reduction of 50% in adult emergence of T. palmi was observed when potting soil with pupae was sprayed with B. bassiana BbHa (Castineiras et al., 1996b). Neozygites parvispora (Zygomycees) has also been documented infecting T. palmi on melons in Japan; approximately 10% of T. palmi adults and nymphs were infected, but the fungus did not control the pest population (Saito et al., 1989). In addition, an unidentified species of the genus Hirsutella (Hyphomycetes) was found in Trinidad, British West Indies, infecting approximately 80% of T. palmi populations in the field (Hall, 1992). 13 6. Chemical control 6.1 UK products Chemical insecticides which are potentially efficacious for thrips control, and are registered in the UK, include: spinosad, abamectin, malathion, nicotine, cypermethrin, deltamethrin, thiacloprid, pirimiphos-methyl smokes, rotenone and bifenthrin, which controls only adults. At the UK label rate, it is reported that teflubenzuron (‘Nemolt’) will suppress but not control thrips (Horticulture Week, 2003). Spinosad and abamectin are recommended for the control of T. palmi in peppers in Hawaii (‘Success’ and ‘Agrimek 0.51 EC’) (Website 1) and Florida (‘Agri-Mek 0.15EC’ and ‘SpinTor 2 SC’) (Maynard et al., 2003). No more than two sequential applications of abamectin are advised, or three applications of spinosad in any 21-day period (Maynard et al., 2003). Spinosad has also been shown to be effective against T. palmi on aubergine (Mau and Gusukuma-Minuto, 1999) and causes no significant mortality in the thrips predator, O. insidiotus (Studebaker and Kring, 1999). It is mainly active against adult and larval thrips, but has some effect on the eggs of selected species. Chemical control of T. palmi in south Florida includes the use of multiple applications of imidacloprid, endosulfan, methomyl and spinosad (Aerts & Mossler, 2000). Other control methods include the release of the native predator, O. insidiosus, and the destruction of old host plants. Best control of T. palmi using spinosad or abamectin is reportedly achieved at first sign of melon thrips infestation, since immature stages are much easier to control (McHugh & Mau, 1998). In the UK, the 3-day pre-harvest interval in cucumbers makes spinosad difficult to use once cropping has begun, although the use of spinosad – which is reportedly compatible with BCAs – might be required in the absence of a tested IPM programme in order to deal promptly with a find of T. palmi. However, the compatibility of spinosad with the BCAs commonly used in cucumber crops needs to be confirmed and the efficacy of Conserve SC – the only spinosad product registered in the UK – against T. palmi on cucumbers, also requires urgent investigation. The Conserve label has no specified rate for T. palmi, just "Thrips (exposed)" in outdoor or greenhouse settings. Examples of exposed thrips are given on the label: i.e. Cuban laurel thrips, Gynaikothrips ficorum and WFT. In other words, this is not a rate for T. palmi. Indeed, the concentration of active ingredient (Spinosyn A and Spinosyn D) in Conserve is only 11.6%, which is almost half, or a quarter, that of products registered in other countries such as the USA: i.e. Success® and SpinTor (both with 22.8% a.i.), Belgium: i.e. Tracer® (48% a.i.) and Spain: SpinTor (48% a.i.). Recommended rates for ‘thrips’ on cucurbit crops are given as 6-8 fl oz/acre in the US and 200ml/ha in Belgium. Consultation with the manufacturer will be required to determine the recommended rate for Conserve against T. palmi, although verification will be required even if the information is available. Field studies have shown that abamectin is highly effective in controlling T. palmi on watermelon, green pepper and aubergine (Mau and GusukumaMinuto, 1999). However, it no longer effectively controls F. occidentalis in UK 14 cucumber crops (Derek Hargreaves, pers. comm.), although the addition of 1% white sugar to the spray mixture has been shown to enhance the kill of thrips larvae by c. 75% (HDC, 2001). Additionally, abamectin has deleterious effects on A. cucumeris and Orius spp., although it could potentially be used in pest ‘hot-spots’ or as an end-of–season clean-up treatment (HDC, 2000b) in a T. palmi eradication programme, if proven effective. Although deltamethrin (‘Decis 2.8% EC’) and cypermethrin (‘5% Cyperkill EC’) have been found to be effective in controlling T. palmi on aubergines in Taiwan (Su et al., 1985), they are broad-spectrum, contact insecticides, the use of which is likely to have a deleterious effect on BCAs and can result in higher populations of T. palmi (Young and Zhang, 1998). Thus, the use of pyrethroids should be avoided, as they may cause melon thrips population explosions (Etienne et al., 1990, Seal & Baranowski, 1992) and whitefly population explosions in UK (Derek Hargreaves, pers. comm.). Of the other broad-spectrum insecticides registered for thrips control in the UK (i.e. malathion, nicotine, pirimiphos-methyl, rotenone and bifenthrin), nicotine is least persistent and BCAs can be re-introduced shortly after application. Therefore, nicotine has the potential to be used as a ‘spot treatment’ in an IPM programme. Thiacloprid has a specific-off label approval for use in protected cucumbers and is effective against F. occidentalis. However, it is detrimental to the parasitoid Encarsia formosa, which is used for whitefly control and is therefore unsuitable for use in IPM in cucumbers (Derek Hargreaves, pers. comm.). 6.2 Overseas products Good results have been reported against T. palmi using methiocarb (Sakimura et al., 1986), insecticidal soaps (e.g. Natrasoap, M-Pede) (Young and Zhang, 1998) and foliar applications of imidacloprid (which are not available in the UK) (Murai, 2001; Cannon et al., in prep.), although resistance to imidacloprid has appeared in some districts in southern Japan (Komi, unpublished data in Murai). Few chemicals have high rates of efficacy against T. palmi, although Murai (2001) reported on a number of older chemicals (mainly organophosphates) which exhibited mortalities of 50% or more – but never more that 80% – against T. palmi: e.g. BPMC, methomyl, fenvalerate, malathion, prothiophos, methidathion and endosulfan. However, these results are based on experience gained in Japan in the late 1970’s and 1980’s, are generally of little applicability to the current UK situation. In Martinique, profenofos, avermectin [spelling for abamectin often used by some countries] and carbofuran were the most effective insecticides against T. palmi on outdoor vegetables (Bon and Rhino, 1989). However, many of the compounds reported as being effective are either not registered for use in the UK (including those which have been revoked or withdrawn), or are unavailable in the formulation required for maximum efficacy. Additionally, there are a number of products, which are only approved for use on ornamental crops in the UK, or are only applicable to soil-based growing 15 systems. For instance, soil treatments with granular formulations of oxamyl and carbosulfan have been shown to depress T. palmi populations (Kawai, 2001), but could not be used in hydroponic cucumbers. Novel insecticides with efficacy against T. palmi include acetamiprid, chlorfenapyr, chlorfluazuron, emamectin benzoate, flucyrthrinate, flufenoxuron and pyriproxyfen. Acetamiprid is a chloronicotinyl insecticide with systemic and translaminar action, which ensures effective control of sucking insects. It has excellent activity against aphids, whitefly and thrips, including T. palmi (Anon, 1998), but is very harmful to Orius spp. (Koppert, Side Effects Guide). Field studies on watermelon, green pepper and aubergine showed that chlorfenapyr was highly effective in controlling T. palmi (Mau and GusukumaMinuto, 1999). Chlorfenapyr will also be a useful component of IPM schemes as it conserves the thrips predator, Orius (Mau and Gusukuma-Minuto, 1999). In Japan, emamectin benzoate (‘Proclaim’) is used for the control of T. palmi in aubergines (Crop Protection Monthly, 1998) and at 5-day intervals, flufenoxuron and chlorfluazuron were the first and third most effective insecticides, respectively in tests against T. palmi in Venezula (Cermeli et al., 1993). Chlorfluazuron (e.g. ‘Aim’, ‘Atabron’) is an insect growth regulator which acts as an anti-moulting agent, leading to the death of thrips larvae and pupae. It is used to control thrips on vegetables (The e-Pesticide Manual, 2002). Flufenoxuron (e.g. ‘Cascade’) is a chitin synthesis inhibitor with contact and stomach action (The e-Pesticide Manual, 2002); it inhibits the ecdysis of first instar T. palmi larvae and metamorphosis of the second instar larvae into pupae, but does not affect the survival rate and fecundity of females. It can be used to supress T. palmi populations (Nagai et al., 1988a). Both chlorfluazuron and flufenoxuron are harmless to nymphs and adults of A. cucumeris, but are very toxic to Orius spp. (Nagai, 1990c; Koppert, Side Effects Guide). Pyriproxyfen could also be a valuable component of an IPM programmes for T. palmi as it controls the pupal stage whilst being harmless to O. sauteri (Nagai, 1990b). Flucythrinate (31.6% ‘Pay-off EC’) was found to be effective in controlling T. palmi on aubergines in Taiwan (Su et al., 1985), but is incompatible with the use of A. cucumeris (Koppert, Side Effects Guide). In conclusion, there are many potentially useful chemicals for T. palmi control, but most are not registered in the UK. Novel compounds with efficacy against thrips in general include azadirachtin, diafenthiuron, ethiprole, fipronil, novaluron, pyridaben and thiamethoxam. A non-systemic liquid formulation of fipronil (‘Vinlin’) is registered in the Netherlands for use on rockwool and soil-based crops, but to date, the only formulation approved in the UK is a soil-applied granule for the control of vine weevil in containerised ornamentals. Pyridaben has both rapid knockdown activity and long residual activity. Due to its low toxicity to beneficial arthropods, it can be included in integrated control programmes (Salagarollo and Politi, 1995). In particular, it has been used in combination with the predatory mite, A. cucumeris, to control F. occidentalis on cucumber and tomato crops (Nawrocka and Szwejda, 1999) and may therefore be able to be used in IPM programmes with similar components against T. palmi. 16 7. Physical control A range of physical control methods, such as mulching with silver PE sheets and the use of UV reflective greenhouses have been tested against thrips (Chu, 1987), but are slow to have an effect and only have a minor impact on populations (Wang et al., 2001). In addition, they are more applicable in situations where the aim is to prevent T. palmi from entering a glasshouse from outdoors, rather than to the eradication of an isolated population. For example, vinyl film, absorbing light < 400nm wavelength reduced T. palmi populations by 30% compared to conventional covering material (Chu, 1987). Mass trapping with sticky traps has been shown to be effective against T. palmi in some crops, and is likely to be used in any T. palmi eradication programme to exert extra pressure on the population, but the indications are that in cucumbers it is only effective against low-density populations. Broad spectrum, contact sprays with a physical mode of action, such as ‘Majestik’ and ‘Eradicoat’, can help to control thrips. They work by blocking spiracles causing suffocation and by coating the outer cuticle preventing movement. Since they act purely by physical means and are not taken into the plant, they can be used on any protected crops, whenever required, with no harvest interval. Their short persistence also makes them compatible with the use of BCAs. They may be applied as an overall spray 24 hours before the introduction of beneficials, or as spot treatments during the growing season. Use of a high volume spray is recommended to achieve the coverage required for effective pest control. 17 8. Recommendations (high priority in bold) 1. Biological Control Agents (BCAs) Laboratory assessments of the efficacy of novel and existing biological products for use in IPM systems against T. palmi, under quarantine conditions, on cucumber plants. Experiments will simulate commercial glasshouse growing conditions, as far as possible, and appropriate methodologies will be developed and tested. A. Prophylactic use of natural enemies I. Host-specific, foliage and flower-inhabiting species a. BCAs already registered in the UK (or in development) 1. Amblyseius cucumeris mites: to determine whether the system currently used in commercial cucumber crops in the UK for controlling WFT – i.e. ‘slow-release’ sachets of A. cucumeris at a rate of 1000 per plant – would successfully control T. palmi. 2. Test other promising thrips predators currently in development, including: A. swirskii A. montdorensis b. BCAs developed specifically for use against T. palmi in countries where it is established (e.g. Japan, Korea, etc.) 1. Compare the efficacy of non-native mites such as Amblyseius asetus, A. mckenziei, A. okinawanus, etc. to that of UKregistered A. cucumeris. 2. Test the predatory thrips, Frankliniella vespiformis c. Generalist ground-dwelling and aerial predators a. b. Anthocoris nemoralis Chrysoperla carnea B. Remedial use of natural enemies: a. BCAs already registered in the UK 1. Orius spp. (Orius laevigatus, Orius majusculus): determine whether these predatory bugs are efficient control agents for T. palmi under realistic conditions. 2. Carry out manipulation experiments (day-length and temperature) to improve the efficacy of Orius spp. 18 b. BCAs developed specifically for use against T. palmi in countries where it is established (e.g. Japan, Korea, etc.) 1. Compare the efficacy of Orius strigicollis vs. UKregistered Orius spp. (O. laevigatus and O. majusculus). 2. Compare the most efficacious Orius spp. with other anthocorids, e.g. Bilia spp., Wollastoniella spp.. c. 2. Insecticides a. 3. Entomopathogenic fungi Spinosad (Conserve SC) 1. Efficacy vs. T. palmi on cucumbers 2. Test wet/dry residues for compatibility with existing BCAs 3. Ensure UK approval would permit sufficient applications and that any pre-harvest interval would allow continuous cropping. 4. Determine the recommended rate against T. palmi for the UK product (Conserve SC). SOLAs and Emergency arrangements CSL to take a strategic approach to securing more T. palmi-effective products; involving consultation with appropriate authorities (PSD, ACRE, etc.) a. Chemicals (SOLAs, Emergency Use Arrangements) b. BCAs (Emergency Use Permits) c. Liaison with biocontrol companies to discuss selection, and ensure availability, of appropriate BCAs for ‘emergency use’. 4. Knowledge of the pest and its impact Improving the existing knowledge-base for T. palmi biology and control on cucumbers and other salad crops Contact Japanese, USA [Hawaii, Florida], Australian [not yet much of a problem] or South America (Venezuela) scientists and growers with experience of controlling T. palmi on cucumbers and other crops. Obtain more information on the economic injury effects of T. palmi in cucumbers from those with experience in affected areas. Revise the CSL PRA for T. palmi 19 5. Integrated pest management and eradication Developing an effective IPM programme for T. palmi integrating all existing knowledge. 1. Contingency plans for dealing with the pest in an outbreak situation under official control, on: i. Cucumbers ii. Peppers iii. Aubergine 2. Longer term IPM programmes in the event of protracted campaigns and spread. 20 9. References Aerts, M. & Mossler, M. 2000. Crop Profile for Eggplant in Florida. http://pestdata.ncsu.edu/cropprofiles/docs/FLeggplant_.html. Alvarez, C.F., Guzman, G.E. and Vergara, R. 2002. Biological features of Thrips palmi (Thysanoptera: Thripidae) and consumption capacity of a natural enemy under laboratory conditions. Revista Colombiana de Entomologia 28(1), 27-31. Anon, 1998. Development of a new insecticide, acetamiprid. Journal of Pesticide Science 23(2), 199-200. Anon. 2003. Pest Control: Blocking a global problem. Horticulture Week. 4 December 2003. Bon, H. de and Rhino, B. 1989. Control of Thrips palmi in Martinique. Agronomie Tropicale 44, 129-136. Brodsgaard, H.H., Sardar, M.A. and Enkegaard, A. 1996. Prey preference of Hyposaspis miles (Berlese) (Acarine: Hypoaspidae): non-interference with other beneficials in glasshouse crops. Bulletin IOBC/WPRS working group on integrated control in glasshouses 19(1): 23-26. CABI, 2004. Crop Protection Compendium. CAB International, Wallingford, Oxon. http://www.cabicompendium.org/cpc/home.asp Cannon, R.J.C. et al. in prep. Thrips palmi outbreak report. Unpublished CSL document. Cannon, R.J.C. 2003. General notes and discussions from the R&D discussion meting on thrips held on Thursday 1st May 2003 at HRI, Wellesbourne. Unpublished CSL document. Capinera, J. L. 2000. Common name: melon thrips; scientific name: Thrips palmi Karny (Thysanoptera: Thripidae). University of Florida. http://creatures.ifas.ufl.edu/veg/melon_thrips.htm. Castineiras, A., Baranowski, R.M. and Glenn, H. 1996a. Temperature response of two strains of Ceranisus menes (Hymenoptera: Eulophidae) reared on Thrips palmi (Thysanoptera: Thripidae). Florida Entomologist 79(1), 13-19. Castineiras, A., Pena, J.E., Duncan, R. and Osborne, L. 1996b. Potential of Beauveria bassiana and Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) as biological control agents of Thrips palmi (Thysanoptera: thripidae). Florida Entomologist 79(3), 458. 21 Cermeli, M. Montagne, A., Godoy, F. 1993. Preliminary results on the chemical control of Thrips palmi Karny (Thysanoptera: Thripidae) on beans (Phaseolus vulgaris L.). Boletin de Entomologia Venezolana 8(1), 63-73. Chambers, R. and Long, S. 1991. A new ally for the grower. Grower June 27 1991. Chambers, R.J., Long, S. and Helyer, N. 1993. Effectiveness of Orius laevigatus for the control of Frankliniella occidentalis on cucumber and pepper in the UK. Biocontrol Science and Technology 3, 295-307. Chu, Y.I. 1987. Physical control of thrips. Proceedings of a Symposium on the Biology of Thrips Chinese Journal of Entomology, Special Publication 1:27-36. ChyiChen, H. and WenHua, C. 2001. Life history and feeding amount of Amblyseius asetus and A. maai (Acari: Phytoseiidae) on Thrips palmi (Thysanoptera: Thripidae). Formosan Entomologist 21(4), 321-328. Cooper, B. 1990. Status of Thrips palmi (Karny) in Trinidad. FAO Plant Prot. Bull. 39(1), 45-46. Cox, P.D., Walters, K.F.A., Matthews, L., Cannon, R. and MacLeod, A. 2004 Potential for the use of biological agents for the control of Thrips palmi outbreaks. Crop Protection Monthly, 1998. Issue 107 – 31st October 1998. www.cropprotection-monthly.co.uk CSL, 2003. Development of a practical IPM approach for the control/eradication of Thrips palmi Karny in the UK. PH0168 – Final Project Report. Cuellar, M.E., Bellotti, A.C. and Melo, E. L. 2002. Aspects of the biology and rate of consumption of Neoseiulus cucumeris and Typhlodromalus aripo (Acari: Phytoseiidae) on the host Thrips palmi Karny (Thysanoptera: Thripidae). Revista Colombiana de Entomologia 28(1), 1-7. Etienne, J., Guyot, J. and Waetermeulen, X. van. 1990. Effect of insecticide, predation and precipitation on populations of Thrips palmi on aubergine (eggplant) in Guadeloupe. Florida Entomologist 73 (2), 339-342. Franz, G. and Mellinger, H.C. 1998. Potential Use of Beauveria bassiana for Biological Control of Thrips in Peppers. Proc. Fla. State Hort. Soc. Paper No. 131. Gillespie, D.R. and Quiring, D.J.M. 1992. Competition between Orius tristicolor (White) (Hemiptera: Anthocoridae) and Amblyseius cucumeris (Oudemans) (Acari: Phytoseiidae) feeding on Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Canadian Entomologist 124: 11231128. 22 Hall, R.A., 1992. New pathogen on Thrips palmi in Trinidad. Florida Entomologist 75: 380-383. Hirose, Y. 1989a. Exploration of natural enemies of Thrips palmi in Southeast Asia. Insitute of Biological control, Faculty of Agriculture, Kyushu University, Fukuoka, 58pp. Hirose, Y. 1989b. Prospective use of natural enemies to control Thrips palmi (Thysanoptera: Thripidae). Institute of Biological Control, Kyushu University, Japan: 135-141. Hirose, Y. 1990. Prospective use of natural enemies to control T. palmi (Thysanoptera: Thripidae). In The Use of Natural Enemies to control Agricultural Pests (J. Bay-Petersen ed.). FFTC, Taipei, pp. 135-141. Hirose, Y., Kajita, M., Takagi, S. Okajima, S., Napompeth, B. and Buranapanichpan, S. 1993. Natural enemies of Thrips palmi and their effectiveness in the native habitat, Thailand. Biological Control 3(1), 1-5. Hirose, Y., Nakashima, Y., Takagi, M., Nagai, K., Shima, K., Yasuda, K. and Kohno, K. 1999. Survey of indigenous natural enemies of the adventive pest Thrips palmi on the Ryukyu Islans, Japan. Applied Entomology and Zoology 34, 489-496. Horticultural Development Council, 2000a. Cucumbers: the development of remedial treatments for the control of western flower thrips within IPM programmes (PC 129). Final report 2000. Horticultural Development Council, 2000b. Protected crops: optimising the use of abamectin within IPM programmes (PC 160). Annual report 2000. Horticultural Development Council, 2001. Protected flower crops: Evaluation of sugar products to improve the control of western flower thrips (PC 177). Final report 2002. Ito, k. and Nakata, T. 1998. Effect of photoperiod on reproductive diapause in the predatory bug, Orius suateri (Poppius) and O. minutus (Linnaeus) (Heteroptera: Anthocoridae). Appl. Entomol. Zool. 33: 115-120. Jacobson, R.J. 1993. Control of Frankliniella occidentalis with Orius majusculus: Experiences during the first full season of commercial use in the U.K. Integrated Control in Protected Crops. Temperate Climate IOBC/wprs Bulletin 16(2) pp. 81-84. Jacobson, R.J. 1995. Egg laying sites of Orius majusculus, a thrips predator, on cucumber. In Parker, B.L. et al. Thrips Biology and Management. Plenum Press, New York. pp. 241-244. 23 Jacobson, R.J. 1997. Integrated pest management (IPM) in glasshouses. In: Thrips as crop pests (Lewis, T. Ed.) CAB International, Wallingford, UK, pp. 639-666. Jacobson, R.J., Chandler, D., Fenlon, J. and Russell, K.M. 2001a. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to Control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on Cucumber Plants. Biocontrol Science and Technology 11: 391-400. Jacobson, R.J., Croft, P. and Fenlon, J. 2001b. Suppressing Establishment of Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in Cucumber Crops by Prophylactic Release of Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae). Biocontrol Science and Technology 11: 27-34. Jacobson, R. J. 2003. PhD Thesis - Implementing Integrated Pest Management in Glasshouses: Challenges and Solutions. Kajita, Y. 1985. Predacious natural enemies of Thrips palmi Karny. Pulex 71, 329-330. Kajita, H. 1986. Predation by Amblyseius spp. (Acarina: Phytoseiidae) and Orius sp. (Hemiptera: Anthocoridae) on Thrips palmi Karny (Thysanoptera: Thripidae). Appl. Ent. Zool. 21 (3): 482-484. Kawai, A. 1995. Control of Thrips palmi Karny (Thysanoptera: Thripidae) by Orius spp. (Heteroptera: Anthocoridae) on greenhouse eggplant. Applied Entomology and Zoology 30: 1-7. Kawai, A. and Kitamura, C. 1987. Studies on population ecology of Thrips palmi Karny. XV. Evaluation of effectiveness of control measures using a stimulation model. Applied Entomology and Zoology 22(3), 292-302. Kirk, W.D.J. (1997) Feeding. In: Thrips as crop pests (Lewis, T. Ed.) CAB International, Wallingford, UK, pp. 119-174. Kohno, K. 1997. Photoperiodic effects on incidence of reproductive diapause in Orius sauteri and O. minutus (Heteroptera: Anthocoridae). Appl. Entomol. Zool. 32: 644-648. Kohno, K. 1998. Thermal effects on reproductive diapause induction in Orius sauteri (Heteroptera: Anthocoridae). Appl. Entomol. Zool. 33: 487-490. Kohno, K. and Hirose, Y. 1997. The Stilt Bug Yemma exilis (Heteroptera: Berytidae) as a Predator of Aphis gossypii (Homoptera: Aphididae) and Thrips palmi (Thysanoptera: Thripidae) on Eggplant. Appl. Entomol. Zool. 32 (2): 406-409. Kumar, N.S. and Ananthakrishnan, T.N. 1984. Predator-thrips interactions with reference to Orius maxidentex Ghauri and Carayonocoris indicus 24 Muraleedharan (Anthocoridae: Heteroptera). Proceedings of the Indian National Science of Academy B 50: 139-145. Kurogi, S., Nakamura, M. and Kawasaki, Y. 1997. Studies on integrated control of major insect pests of sweet pepper in a greenhouse [in Japan]. 3. Control of Thrips palmi with 2 species of predators, Orius sauteri and Amblyseius cucumeris. Proceedings of the Association for Plant Protection of Kyushu 43: 106-109. Lenteren, J.C. van. 1995. Integrated pest management in protected crops. In: Integrated Pest Management (DENT, D. Ed.) Chapman and Hall, London, UK, pp.311-343. Loomans, A.J.M. and Heijboer, A. 1999. Frankliniella vespiformis (Crawford): a polyphagous predator preying on thrips eggs. Proceedings of Experimental and Applied Entomology, The Netherlands Entomological Society (N.E.V.) 10: 143-150. Loomans, A.J.M. and Vierbergen, G. 1999. Franklinothrips: perspectives for glasshouse pest control. IOBC/WPRS working group on integrated control in glasshouses 22 (1), 157-160. John J. McHugh, Jr. & Ronald F. L. Mau 1998. Pepper. Table 2. Insect and Mite Control Recommendations. http://www.extento.hawaii.edu/kbase/reports/recommendations/peppers.asp Mau, R.F.L., Johnson, M.W., DeFrank, J.J., Welter, S.C. 1989. Biological analysis of Thrips palmi in the Pacific Basin. In: Tropical and subtropical agricultural research under PL 89-106, special research grants. Progress and achievements, the Pacific Basin group, 1989, p.15. Mau, R.F.L. and Gusukuma-Minuto, L. 1999. Insecticidal management of key thrips pests of fruiting vegetables, onions and corn in Hawaii. Proceedings: Sixth International Symposium on Thysanoptera, Akdeniz University, Antalya, Turkey 27th April-1st May, 1998. Akdeniz University, Faculty of Agriculture, Department of Plant Protection, Antalya, Turkey: 1999. 107-112. 8 ref. Maynard, D.N., Hochmuth, G.J., Vavrina, C.S., Stall, W.M., Kucharek, T.A., Webb, S.E., Taylor, T.G., Smith, S.A., Simonne, E.H. and Olson, S.M. 2003 Chapter 34. Pepper Production in Florida. Document HS732, horticultural Sciences Dept., UF/IFAS, Florida Cooperative Extension Services, September 2003. Murai, T. 2001. The pest and vector from the East: Thrips palmi. Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera: Thrips, Plants, Tospoviruses: the Millennial Review, Reggio Calabria, Italy, 2nd - 7th July 2001. 25 Nagai, K., Hiramatsu, T. and Henmi, T. 1988a. Effect of flufenoxuron on Thrips palmi Karny. Japanese Journal of Applied Entomology and Zoology 32(4), 297-299. Nagai, K., Hiramatsu, T. and Henmi, T. 1988b. Predatory effect of Orius sp. (Hemiptera: Anthocoridae) on the density of Thrips palmi Karny (Thysanoptera: Thripidae) on eggplant. Japanese Journal of Applied Entomology and Zoology 32: 300-304. Nagai, K. 1990a. Suppressive effect of Orius sp. (Hemiptera: Anthocoridae) reared on Thrips palmi Karny (Thysanoptera: Thripidae). Japanese Journal of Applied Entomology and Zoology 34: 109-114. Nagai, K. 1990b. Effects of a juvenile hormone mimic material, 4 – phenoxyphenyl (RS) – 2 – (2 – pyridyloxy) propyl ether, on Thrips palmi Karny (Thysanoptera: Thripidae) and its predator Orius sp. (Hemiptera: Anthocoridae). Applied Entomology and Zoology 25: 199-204. Nagai, K. 1990c. Effect of insecticides on Orius sp., the natural enemy of Thrips palmi Karny. Japanese Journal of Applied Entomology and Zoology 34(4), 321-324. Nagai, K. 1993a. Integrated pest management of Thrips palmi Karny in eggplant fields. Proceedings International Symposium on the “Use of biological control agents under integrated pest management”. Fuukuoka, Japan, October 4-10, 1993, 384-404. Nagai, K. and Yano, E. 1999. Effects of temperature on the development and reproduction of Orius sauteri (Poppius) (Heteroptera: Anthocoridae), a predator of Thrips palmi Karny (Thysanoptera: Thripidae). Appl. Entomol. Zool. 34: 223-229. Nagai, K. and Yano, E. 2000. Predation by Orius sauteri (Poppius) (Heteroptera: Anthocoridae) on Thrips palmi Karny (Thysanoptera: Thripidae): functional response and selective predation. Appl. Entomol. Zool. 35: 565574. Nakashima, Y., Uefune, M., Tagashira, E. Maeda, S., Shima, K., Nagai, K., Hirose, Y. and Takagi, M. 2004. Cage evaluation of augmentative biological control of Thrips palmi with Wallastoniella rotunda in winter greenhouses. Entomologia Experimentalis et Applicata 110, 73-77. Nawrocka, B. and Szwejda, J. 1999. Influence of soilless cultures on development and control of western flower thrips, Frankliniella occidentalis (Pergande) occurring on glasshouse vegetable crops. Vegetable Crops Research Bulletin 50, 47-54. Paik, C-H., Hwang, C-Y., Lee, G-H., Kim, D-H., Choi, M-Y., Na, S-Y. and Lim, S-S. 2003. Development, reproduction and longevity of predator Orius sauteri 26 Poppius (Hemiptera: Anthocoridae) when reared on three different prey. Korean J. Appl. Entomol. 42(1): 35-41. Parker, B.L., Skinner, M. and Lewis, T. 1995. Predatory capacity of Campylomma chinensis Schuh (Hemiptera: Miridae) and Orius sauteri (Poppius) (Hemiptera: Anthocoridae) on Thrips palmi. Thrips biology and management: Proceedings of the 1993 International Conference on Thysanoptera. Plenum Publishing Co. Ltd, London, USA: 1995. 259-262. Rosenheim, J.A., Welter, S.C., Johnson, M.W., Mau, R.F.L. and Gusukuma Minuto, L.R. 1990. Direct feeding damage on cucumber by mixed-species infestations of Thrips palmi and Frankliniella occidentalis. Journal of Economic Entomology 83,1519-1525. Ruberson, J.R., Bush, L. and Kring, T.J. 1991. Photoperiod effect on diapause induction and development in the predator Orius insidiosus. Environmental Entomology 20(3), 768-780. Saito, T., Kubota, T. and Shimazu, S. 1989. A first record of the entomopathogenic fungus, Neozygites parvispora (MacLeod and Carl) Rem. and Kell., on Thrips palmi Karny (Thysanoptera: Thripidae) in Japan. Applied Entomology and Zoology 24: 233-235. Saito, T. 1992. Control of Thrips palmi and Bemisia tabaci by a mycoinsecticidal preparation of Verticillium lecanii. Proceedings of the Kanto Tosan Plant Protection Society. 39: 209-210. Sakimura, k., Nakahara, L.M. and Denmark, H.A. 1986. A thrips, Thrips palmi Karny. Fla. Dept. Agric. and Consumer Serv. Division of Plant Industry. Entomology Circular No. 280 4pp. Salagarollo, V. and Politi, A. 1995. Pyridaben (Nexter ®): a new acaricide. Informatore Fitopatologico 45(5), 28-32. Seal, D.R. and R.M. Baranowski. 1992 Effectiveness of different insecticides for the control of melon thrips, Thrips palmi Karny (Thysanoptera: Thripidae) affecting vegetables in south Florida. Proc. Fla. State Hort. Soc. 105: 315319. Searca, S. 1991. Management of thrips and mites attacking potato in the lowland. SEAMEO Research and Development Programme. SEAMEO Quarterly 14 (4), 41-43. Shimizu, T. and Kawasaki, K. 2001. Geographic variability in diapause response of Japanese Orius species. Entomol. Exp. Appl. 98: 303-316. Shipp, J.L. and Ramakers, P.M.J. 2000. Biological control of thrips in protected greenhouse vegetables. In Heinz, K.M., Driesche, R.G. van and Parrella, M.P. Biological Control in Protected Culture. Ball Publishing, Chicago. 27 Song, J-H., Kim, S-N., Lee, K-S and Han, W-T. 2002. Analysis of Spatial Coincidence of Thrips and Orius sauteri on Greenhouse Eggplants. Korean J. Appl. Entomol. 41(1): 27-32. Studebaker, g.E. and Kring, T.J. 1999. Lethal and sub-lethal effects of selected insecticides on Orius insidiosus. 1999 Proceedings Beltwide Cotton Conferences, Orlando, Florida, USA, 3-7 January 1999: Volume 2. National Cotton Council, Memphis, USA: 1999. 1203-1204. Su, C.Y., Chiu, T.S. and Lin, Y.J. 1985. Study of population fluctuation of Thrips palmi and its insecticidal control in the field on eggplant. Chinese Journal of Entomology 5(2), 101-118. Try, Y. and Hung, H. Q. 2003. Composition of natural enemies (parasitoids, predators) of Thrips palmi Karny attacking on Soybean. Tap chi KHKT Nong nghiep, Tap 1. Wada, T. 1999. Development of Cucumeris ® and its future prospect. Agrochemicals Japan. No. 73, 17-19. Walker, A.K. 1994. A review of the pest status and natural enemies of Thrips palmi. Biocontrol News and Information 15(1): 7N-10N. Wang, C.L. 1994. The predacious capacity of two natural enemies of Thrips palmi Karny, Camplylomma chinensis Schuh (Hemiptera: Miridae) and Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Plant Protection Bulletin (Taiwan) 36: 141-154. Wang, C-L., Lee, P-C., and Wu, Y-J. 2001. Field augmentation of Orius strigicollis (Heteroptera: Anthocoridae) for the control of thrips in Taiwan. http://www.agnet.org/library/article/eb500.html Wei, C.S., Peng, Z.J., Yang, G.Q., Cao, Y., Huang, B.Z. and Chen, X. 1984. Natural Enemies of Insects 6: 32-40. Welter, S.C., Rosenheim, J.A., Johnson, M.W., Mau, R.F.L. and Gusukuma Minuto, L.R. 1990. Effects of Thrips palmi and western flower thrips (Thysanoptera: Thripidae) on the yield, growth, and carbon allocation pattern in cucumbers. Journal of Economic Entomology 83:2092-2101. Yano, E. Recent progress in integrated pest management of vegetables. Yano, E. 1999. Recent advances in the study of biocontrol with indigenous natural enemies in Japan. IOBC/WPRS Bulletin 22(1): 291-294. Yano, E. 2003. Biological control of vegetable pests with natural enemies. APO Bulletin, Asian Productivity Organization, Tokyo. 28 Yano, E. 2004. Recent development of biological control and IPM in greenhouses in Japan. Journal of Asia-Pacific Entomology 7(1): 5-11. Yasunaga, T. 1995. A new species of the genus Wooastoniella, predator of Thrips palmi in aubergine gardens of Thailand. Applied Entomology and Zoology 30, 203-205. Yasunaga, T. 1997. The flower bug genus Orius Wolff (Heteroptera: Anthocoridae) from Japan and Taiwan, Parts I, II, III. Appl. Entomol. Zool. 32: 355-364, 379-386, 387-394. Young, G. and Zhang, L. 1998. Control of melon thrips, Thrips palmi. Agnote 753. No. I45. Agdex 230/612. Department of Primary Industries and Fisheries, Northern Territory. www.nt.gov.au/dbird/dpif/pubcat Websites 1 http://www.extento.hawaii.edu/kbase/reports/recommendations/pepper 2 http://pest.cabweb.org/Archive/Pestofmonth/n-thripl.htm 3 http://www.agrofrontier.com/staff/development.html 4 http://www.novartis-agri.com/ 5 http://www.koppert.nl/e005.shtml 6 http://www.agrofrontier.com/staff/development.html 29 Appendix 1 Cucumber Grower Association Proposed Strategy for the Management of Thrips palmi in Cucumber Crops 30 A Proposed Strategy for the Management of Thrips Palmi in Cucumber Crops The Purpose of the Cucumber Growers’ Association: The CGA exists to look after the collective interests of the UK industry and, where possible, anticipate and prepare for new problems. CGA’s approach to pest control: UK cucumber growers currently combat up to 14 pest species with a sophisticated integrated pest management (IPM) programme. If we consider the ultimate goal in pest control to be the cost-effective production of adequate supplies of high quality produce in the absence of synthetic chemical toxins, then UK cucumber growers are among the most advanced practitioners in the world. It is a tribute to the whole industry that, despite many new challenges over the last 15 years, they have remained dedicated to the principles of IPM and have become even more focused on their quest to eliminate chemical pesticides from their production systems. Importance of IPM to marketing British cucumbers: Throughout the 1990s, the major UK retailers responded to consumer fears about pesticide residues in food by introducing Codes of Practice for their suppliers, which set demanding standards for food production including restrictions on pesticide usage. UK cucumber growers rose to this challenge and have developed good relationships with retailers despite increasing competition from overseas. The latter, and in particular Mediterranean growers, still tend to use routine chemical pest control strategies based on relatively inexpensive products that are often not available in this country. Although this provides them with a financial advantage, it is not what the customer ultimately wants, and it is therefore vital that UK growers continue to build on their IPM advantage. One means of doing this is to perfect methods of growing all year round crops that will reduce the retailers’ dependence on Mediterranean produce between October and February. There is no doubt that the continued use of IPM is vital to the future of the UK cucumber industry. Threat posed by Thrips palmi The CGA are aware of the threat posed by Thrips palmi to the UK industry. We are also aware of the approaches taken by Plant Health when outbreaks of this pest have 31 occurred on individual cucumber and cut flower nurseries in the past. Our current pest control programme is heavily dependent on biological control agents and we are very concerned that the use of broad spectrum chemical pesticides against Thrips palmi will prevent us from continuing to use this strategy. An effective IPM strategy for the control Thrips palmi is therefore considered to be high priority. A sustainable strategy for the control of Thrips palmi In nature, populations of herbivorous pests are usually constrained by a range of natural enemies that attack different life cycle stages and/or operate at different prey population densities. Similarly, biologically-based control programmes in permanent and semipermanent agricultural ecosystems are most successful in maintaining pest populations below economic damage thresholds when the natural enemy biodiversity is high and when a large proportion of the pest’s life cycle is exposed to those natural enemies. It is proposed that more sustainable biologically-based control programmes against Thrips palmi will similarly depend on a suite of natural enemies with complementary life styles. This presents a challenge because the life cycle stages of Thrips palmi are found in so many different locations within the crop habitat. Multiple natural enemy approaches to Thrips palmi control could operate at four levels, which would ideally be based on a foundation of host plant resistance: 1. prophylactic use of foliage and flower inhabiting natural enemies 2. prophylactic use of generalist natural enemies on the ground 3. remedial use of invertebrate natural enemies 4. remedial use of entomopathogenic fungi There are many natural enemies available that could be complementary within these four levels. The CGA’s strategy proposes that these individual control measures be developed as independent modules that can then be put together in different combinations to suit different demands (eg pest eradication or management). Benefits to other horticultural crops: The modules developed for the control of Thrips palmi on cucumbers could subsequently be assembled in combinations that are appropriate to other crops that are vulnerable to the pest. In other words, the cucumber crop could be used as a model experimental system for the ultimate benefit of all horticultural crops. Derek Hargreaves, CGA Technical Officer Rob Jacobson, CGA Secretary On behalf of CGA Main Committee 32 Appendix 2 A modular approach to integrated control of Thrips palmi Karny: Concept note 33 A MODULAR APPROACH TO INTEGRATED CONTROL OF THRIPS PALMI KARNY Concept note Contact: Keith Walters, Central Science Laboratory, Sand Hutton, York, YO41 1LZ Tel: 01904 462203; email: k.walters@csl.gov.uk Consortium: Central Science Laboratory (CSL; Research participant), Stockbridge Technology Centre (STC; Research participant); Cucumber Growers Association (CGA; Commercial advisors) Background Thrips are serious crop pests throughout the world causing damage by both direct feeding and their ability to transmit viruses. Following crop colonisation their thigmotactic caustral behaviour can make them difficult to detect, short generation times result in rapid population increases and development of insecticide resistance can result in control failures. In recent years several species of thrips have been dispersed globally as a result of their association with the international trade in growing plants or plant produce. Thrips palmi Karny was known to be widely distributed in Sudan and Taiwan before suddenly becoming a pest in Japan in 1978, since when it has spread throughout the world. It is a very polyphagous pest, which can cause significant economic losses in countries where it establishes. Pest Risk Assessments indicate the species represents a serious threat to the UK protected horticulture industry, which must therefore be provided with methods to contain and eradicate it. The limited range of chemical insecticides that are available for the control of T. palmi in Europe, the development of insecticide resistance and increased public concern about the potential effects of such pesticides on the environment, underline the need for non-chemical approaches to thrips control to be developed. In addition, the consequential trend in several sectors of the horticultural industry towards reduced or no-chemical production systems reinforces this requirement. Following the successful development and use of biological control agents (BCAs) as components of integrated pest management (IPM) approaches designed to eradicate quarantine leafminers (Liriomyza huidobrensis), the initial promise of similar investigations for the whitefly Bemisia tabaci, and against the background of research into IPM strategies for F. occidentalis, research has been undertaken into the potential for using IPM against T. palmi (project PH0168). Laboratory experiments were used to investigate an approach that utilises both chemical and non-chemical (entomopathogenic fungi and nematodes). The results established the potential of these non-chemical agents for the control and eradication of T. palmi but indicate that to be used successfully they must be incorporated into a planned programme that combines them with other classes of control agents or with conventional pesticides. Ideally, the system should include two levels of action. a. Prophylactic use of BCAs to reduce the potential of establishment in commercial crops of accidentally introduced T. palmi (or where an outbreak has been detected to reduce/prevent spread to adjacent glasshouses/holdings). b. Use of BCA s and/or chemical control agents to eradicate T. palmi outbreaks. The polyphagous nature of T. palmi make it difficult to predict with accuracy which UK crops are most at risk of infestation by the species. Accordingly any control strategy devised must be adaptable for use on a range of commodities. This presents further difficulties in using BCAs as part of a control strategy, as most such agents are known to be effective on a limited range of host plants or growing conditions. The solution is to use a commercially important at-risk crop as a model for the development of the strategy but to ensure that the control 34 agents selected for use on the model crop have equivalents that are appropriate for use on other commodities. Cucumber is a major UK horticultural crop at-risk of damage by T. palmi. UK cucumber growers have already adopted IPM against all the pests that commonly attack their crops but the programme is currently dependent on occasional use of dichlorvos to regulate existing thrips’ pests (largely Frankliniella occidentalis). This OP insecticide is currently under review by the Advisory Committee on Pesticides and could be lost to the industry in the near future. Furthermore, to survive in the global market, the Cucumber Growers’ Association (CGA) recognise that they must adapt their production practices and have therefore set research priorities that include pesticide free production, continuous year-round production using supplementary lights and increased production of soil-grown organic crops to satisfy the home market. The need to utilise chemicals for the eradication of quarantine organisms such as T. palmi is not compatible with the CGA’s objectives and strategies must be developed that minimise the number of occasions on which this will be necessary in the event of an outbreak. Cucumber is thus a strong contender as a model crop for research into IPM for T. palmi that will be used in the proposed research. Approach From the published literature and results of project PH0168 it has become clear that reliable non-chemical control of T. palmi will not be achieved by simply releasing greater numbers of the BCAs that are currently in commercial use. More sustainable control will inevitably depend on a suite of natural enemies with complementary life histories and behaviour and this must be built on a solid foundation of knowledge of the tritrophic interactions between plants, pests and natural enemies. Recent work in experimental cucumber crops has shown that a combination of prophylactic and remedial control measures provides a basis for robust control programmes that would also be appropriate for a wide range of other crops. In nature, herbivorous invertebrates are usually constrained by a range of natural enemies that attack different life cycle stages and/or operate at different prey population densities. A more sustainable IPM package against the important thrips pests of protected crops will similarly depend on a suite of natural enemies with complementary life styles. The different life cycle stages of T. palmi may be found in different locations within the crop habitat and biological control of T. palmi is likely to be enhanced by maximising the proportion of the pest’s life cycle that is exposed to natural enemies. It is anticipated that this will operate at four levels: a. b. c. d. prophylactic use of foliage and flower inhabiting natural enemies prophylactic use of generalist natural enemies in the surface layer of soil use of invertebrate natural enemies to eradicate use of entomopathogens to eradicate A suite of control modules involving different control agents will be developed for each level. Those modules best suited to particular crops can then be selected and integrated into an optimum control strategy for that crop which maximises the control exerted at all levels. The principles underpinning the approach will be researched and established using cucumbers, but the potential for extrapolation to named ornamentals will be assessed. Thus the system will be generic for all protected crops. Potential BCAs for the control of T. palmi A range of potential BCAs for use against T. palmi can be identified in the literature including: Insect and mite predators:- Aeolothripidae: Franklinothrips vespiformis; Anthocoridae: Bilia sp.; Carayonocoris indicus; Orius armatus; Orius insidiosus; Orius maxidentex; Orius minutus; Orius nagaii; Orius sauteri; Orius similis; Orius strigicollis; Orius tantillus; 35 Wollastoniella parvicuneis; Wollastoniella rotund; Berytidae: Yemma exilis; Cecidomyiidae: Arthrocnodax occidentalis; Chrysopidae: Mallada basalis; Coccinellidae: Coleomegilla maculata; Propylea japonica; Lygaeidae: Geocoris lubra; Geocoris ochropterus; Piocoris varius; Stigmatonotu minutum; Miridae: Campylomma austrina; Campylomma chinensis; Campylomma livida; Deraeocoris; Orthotylus; Phlaeothripidae: Haplothrips victoriensis; Phytoseiidae: Amblyseius cucumeris; Amblyseius longispinosus; Amblyseius mackenzie; Amblyseius multidentatus; Amblyseius okinawanus; Amblyseius tsugawai; Phytoseius spp.; Thripidae: Neohydatothrips portoricensis. Parasitoids: Trichogrammatidae: Geotheana shakespearei. Megaphragma spp.; Eulophidae: Ceranisus menes; Fungal pathogens:- Hyphomycetes: Beauvaria bassiana; Cladosporium cladosporioides; Hirsutella sp.; Verticillium lecanii; Zygomycetes Neozygites parvispora. Nematodes:- Steinernematidae: Steinernema feltiae These species will be assessed for their potential as components of the above control levels on different crop types and a sub set selected for detailed study. Integration of chemical insecticides There are two methods of integrating chemicals into IPM programmes; either as highly selective products that are specific to the target pest or less specific products that may be applied in a manner, or at a time, which has minimal effect on natural enemies on the crop. There are currently no truly selective insecticides available for thrips, so control measures are limited to careful use of more broad spectrum products. Chemicals approved for thrips species such as T. palmi that may have an important role in chemical control programmes and could also be a useful addition to the IPM armoury for both ornamental and edible crops will be tested for compatibility with the selected BCAs. Further studies will also be required to determine how this insecticide can be utilised in IPM programmes. Mutual compatibility of control agents In IPM programmes that utilise multiple control measures, optimisation of each agent relies upon a sound knowledge of its compatibility with the others. For example, the potential for utilising two agents in parallel or the interval required between application/release of two antagonistic agents must be established. Initial screening techniques developed by one of the partner laboratories to compare mutual compatibility of biological control agents will be employed to establish optimal selection ands use of agents in each of the modules of the IPM system. Objectives To take advantage of previous research findings to further develop IPM-based control/eradication programmes against T. palmi in key glasshouse crops within the constraints of a finite budget by: 1. Evaluating the potential for control of T. palmi of existing and novel biological control agents, within the following categories: a. prophylactic use of foliage and flower inhabiting natural enemies of suppress population development and reduce establishment potential b. prophylactic use of generalist natural enemies in the surface layer of soil to suppress population development and reduce establishment potential c. use of invertebrate natural enemies to eradicate T. palmi populations d. use of entomopathogens to eradicate T. palmi populations 2. Establishing compatibility of selected BCAs with chemical insecticides used in the host crop, and mutual compatibility of the selected BCAs 36 3. Evaluating the potential advantages of using the selected control agents in combination rather than individually in the above categories. 4. Designing, evaluating and refining preliminary modular-based control strategies for the control of T. palmi under quarantine cage conditions 5. Assess the potential for using the modular-based approach as an IPM strategy in other crops. Work plan 1. Organise a project steering Group incorporating representatives of PHD, PHSI, Growers and CSL PH consultants. Objective 1: 2. Undertake a literature search to determine the full range of potential BCAs for T. palmi control and select candidate species for further research. Prophylactic use of foliage and flower inhabiting natural enemies: 3. Collate existing knowledge of selected control agents, identify relevant gaps and instigate small-scale experimentation to complete performance data packages. 4. Where available, test the use of existing application technology for each control agent under quarantine conditions (e.g. culture pack systems). 5. Prepare prototypes of new systems as necessary, determine how long the cultures remain active and quantify their output compared to any standard system that is currently used in cucumber crops, and make any modifications necessary to improve performance. 6. Refine the selection of control agents if appropriate. Prophylactic use of generalist natural enemies in the surface layer of soil 7. Collate existing knowledge of selected control agents, identify relevant gaps and instigate small-scale experimentation to complete performance data packages. 8. Quantify the performance of selected agents in various growing media (e.g. soil, organic compost, peat based growing media). 9. Establish application protocols. 10. Refine the selection of control agents if appropriate. Use of invertebrate natural enemies to eradicate T. palmi populations 11. Collate existing knowledge of selected control agents, identify relevant gaps and instigate small-scale experimentation to complete performance data packages. 12. Refine the selection of control agents if appropriate. 13. Establish application protocols. Use of entomopathogens to eradicate T. palmi populations 14. Collate existing knowledge of selected control agents, identify relevant gaps and instigate small-scale experimentation to complete performance data packages. 15. Refine the selection of control agents if appropriate. 16. Establish application protocols Objective 2: 17. Conduct small scale experiments to establish the compatibility of the selected BCAs within each usage category. 18. Conduct small scale experiments to establish the compatibility of selected BCAs with appropriate chemical insecticides. 19. Refine the selection of control agents if appropriate. Objective 3: 37 20. Within each usage category and where appropriate, conduct small scale experiments to establish the impact of simultaneous use of BCAs and compare with the impact of using them individually. Objective 4: 21. Where appropriate integrate the findings of objectives 1 - 3 into protocols for the effective use of the selected control agents against T. palmi. 22. Using these protocols as a basis, design, evaluate and refine preliminary modularbased control strategies for the control of T. palmi in cucumber crops. Ensure that protocols that both include and do not include the use of chemical insecticides are drafted. 23. If possible, test protocols using cage experiments under quarantine containment. Objective 5: 24. Assess the potential for extrapolating the protocols into other at-risk crops 25. Present protocols for all modules and theoretical programmes to the steering group and predict performance in key crops (i.e. cucumber, chrysanthemum). 26. Estimate the parameters within which the agents will be successful in each situation. 27. Select combinations of complementary modules with potential to form part of a biological control programmes for each crop. Produce theoretical biological control programmes for selected key crops. 28. Prepare cost-benefit analyses of biological control programmes. (STC) Benefits Difficulties relating to the withdrawal of active ingredients and products from the market, reluctance of manufacturers to seek registration approval for the use of new products on commodities representing a small proportion of the worldwide cropping area (e.g. many horticultural crops) and public concern over environmental issues, result in the need for improved biocontrol/IPM measures for quarantine pests. The research described in this proposal represents the first investigation of a flexible modular approach to the use of biological and chemical agents for the control of a major quarantine glasshouse pest. The treatments developed would be suitable for nurseries that have existing biocontrol programmes used in conjunction with chemical pesticides, as well as for those in organic production. This project would also enable protocols for management strategies to be drawn up that could be tailored to the constraints of both the ornamental and vegetable industries, which have markedly different requirements. The inclusion of Plant Health consultants in the delivery team will ensure that the work is directed at quarantine issues of immediate concern and takes account of unique considerations and constraints relating to requirements for containment and eradication of quarantine pests such as T. palmi. Additionally PHD, PHSI, CSL, consultants and growers will obtain further benefits because of the technology transfer framework that will be utilised by the project for optimal delivery of results to those who need them. It is anticipated that output from components of the research programme could be delivered to the PHSI for immediate field application before the end of the project. The findings will also provide background information to assist decisions on the need for and appropriate targeting of future research. More widely, the results of this project will reduce the UK protected crop industry’s dependence on chemical insecticides, thus contributing to Defra’s aims and objectives by providing sustainable pest management programmes based on a combination of compatible and complementary biologically based control measures and improving the competitiveness of the UK industry by satisfying retailer and consumer requirements for a reduction in the amount of chemical toxins used within the produce supply chain. Cost: £73K per annum for four years 38