01 Important Concepts
advertisement

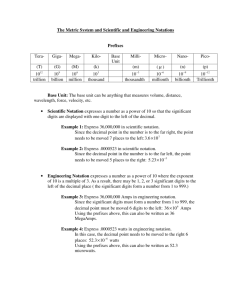
9 Introduction and Review of Some Important Concepts Got Moles? 10 ... there is poetry in science, but also a lot of bookkeeping. Peter Medawar, “Two Conceptions of Science” (1965)1 1 in Peter Medawar, Pluto’s Republic. Oxford: Oxford University Press, 1984, p. 32. 11 I. Significant Figures A. Exact Numbers and Measured Numbers In chemistry, the numbers that we encounter usually may be categorized either as exact numbers or as measured numbers. Exact numbers are relationships that are true by definition. Examples include 12 inches = 1 foot, 5280 feet = 1 mile, 60 seconds = 1 minute, or 1000 millimeters = 1 meter. There is no uncertainty in these numbers, and they have an infinite number of significant figures. Measured numbers, on the other hand, describe a property of an object such as its weight, volume, length, etc. They are determined by one or more laboratory experiments and require the use of a measuring instrument such as a ruler or a balance. A measuring device never gives the exact numerical value of that property; there is always some uncertainty in the value. There is therefore a limit to how precisely these numbers are known, and there is a limit to the number of significant figures contained in the number, which must be taken into account when doing calculations based on that number. For example, suppose you wish to measure the mass of an object in grams using a balance whose read-out looks like this: The pointer has settled between 13 and 14 grams, so you know that the weight is 13 grams plus a fraction of a gram. However, there are no marks on the scale between 13 and 14. We might record this weight as 13.3 grams if we thought that the pointer was approximately 3/10 of the way between 13 and 14. It is important to note that the last digit is an estimate of the numerical value of that position — trying to estimate any more decimal places than that would be a wild guess. Even if there had been marks between the 13 and the 14, the pointer could still point somewhere in between those marks, and we would still have to estimate the last digit in the reading, this time in the hundredths place (0.01) instead of the tenths place. When reading a scale, you should record all of the digits that you are sure of, plus one additional estimated digit. This estimated digit is the last significant figure in your reading. (On a digital readout, the last number on the screen is usually the last significant figure.) 12 B. Counting Significant Digits The concept of significant digits describes how accurately a number has been measured, and thus applies only to measured numbers, not to exact numbers. To determine the number of significant digits in any measured number, we count every digit whose value we know for certain, plus one estimated digit. Digits that serve solely to establish the size of a number (leading zeroes or trailing zeroes) do not count as significant. The following rules and examples will help you to determine the number of significant digits in any number which you or anyone else has written. Number a. All non-zero digits are significant. 164.87 395 Significant Digits 5 3 Zeros may indicate either a digit value or the size of the number, and require separate rules: b. Leading zeros are never significant. 0.766 0.000033 00591.3 3 2 4 c. Middle zeros are always significant. 2.028 5107 0.00304 4 4 3 d. Trailing zeros are significant if the number contains a decimal point. 14.30 0.0030 500. 500.0 4 2 3 4 e. Trailing zeros are ambiguous if the number contains no decimal point. We usually assume that they are not significant 2500 60 2 (or, at least 2) 1 (or, at least 1) One way to indicate that trailing zeros are significant is to place a bar over the last significant zero, for example 2500 (3 significant digits), or 2500 (4 significant digits). An alternate way is to report the numbers in scientific notation (2.5103 = 2 sf’s, 2.50103 = 3 sf’s, 2.500103 = 4 sf’s). It is important to recognize that calculators do not know anything about significant digits! This is especially important when performing arithmetical operations on measured numbers, in which some of the digits must be discarded because they are not significant. 13 C. Calculations Involving Significant Figures Usually we measure certain properties of an object, and calculate other properties from those measurements. For example, we may measure the length and width of a rectangle and calculate its area by multiplying its length by its width or calculate its perimeter by adding twice its length to twice its width. Since the accuracies of the measured length and width were limited, the accuracy of the calculated area and perimeter must also be limited. No calculated number can be any more accurate than the least accurate number used to perform the calculation. For example, it makes no sense to report the mileage in the calculation below to over ten decimal places, when the numbers that went into the calculation contained four significant figures: Mileage Miles 355.4 mi 31.45132743363 ... mi/gal Gallons 11.30 gal We determine the limit on the number of significant figures using two rules, depending on whether the calculation involves (1) multiplication/division or (2) addition/subtraction. Each technique requires that we be able to round off numbers correctly. Rounding Off. Rounding off a number amounts to reducing the number of significant digits in that number. When rounding off a number to an appropriate number of significant figures, look at the digit immediately to the right of the last significant digit. If the digit being discarded is less than 5, the last significant digit is not changed. If the digit being discarded is 5 or greater, the last significant digit is rounded up by one. In the examples below, each of the numbers is rounded off to three significant digits: Original Number 122.6 0.15328 6379.54 0.00078456 1.275 6,143,781 Number Rounded to Three Significant Figures 123 0.153 6380 0.000785 1.28 6,140,000 Addition and Subtraction. When adding or subtracting, the answer may not contain significant digits further to the right than those in any of the numbers being added or subtracted. In other words, the place in which the significant figures stop first is where the significant figures stop in the answer. For example: 6.14 + 0.0375 6.1775 rounds off to 6.18 5200 + 63.4 5263.4 rounds off to 5260 0.002631 – 0.0014278 0.0012032 1.230 + 12.270 13.500 rounds off to 0.001203 14 Notice that in the last example, the final answer is reported as “13.500” (5 sf’s), not 13.50 (4 sf’s) or 13.5 (3 sf’s). The final zero in the third decimal place is significant, because the measurements contained significant digits in the third decimal place. The zero in the second decimal place is significant, because it came from two numbers in the second decimal place adding together to give a “10.” Multiplication and Division. When multiplying or dividing, the answer may not contain more significant digits than any of the numbers being multiplied or divided. In other words, the number with the smallest number of significant figures determines the number of significant figures in the answer. For example: 5.77 3.036842105263... 1 .9 rounds off to 3.0 (2 sf’s) (28.71)(0. 0626)(128.54) 46.203600168... (5.0) rounds off to 46 (2 sf’s) Raising to powers and taking roots follow the same rules as multiplying/dividing. (2.05)2 = 4.2025 rounds off to 4.20 (3 sf’s) (2.25)(4.9) 3.320391543177... rounds off to 3.3 (2 sf’s) Losing Significant Figures. Sometimes you can lose significant digits when subtracting. For example: 12.6198 (6 significant digits) – 12.5202 (6 significant digits) 0.0996 (3 significant digits) You cannot “invent” significant figures past the fourth decimal place to make up for the number of significant figures lost, because no measurements were made past the fourth decimal place. Occasionally the rules may be bent slightly. In the example above, since 0.0996 is so close to 0.1000 (4 sf’s), you probably could consider 0.0996 to have 4 significant figures if you were to use it in a multiplication or division. Combined Operations. When operations involving both addition/subtraction and multiplication/division are performed, the order of operations is important when determining the number of significant figures in the final answer: 500.00 500.00 10.0 275.0 - 225.00 50.0 (3 significant figures, based on the subtraction step in the denominator) 10.00×10.00 – 200.0/20.00 (one decimal place, based on the fact that the significant figures stop at the first decimal place in the first part of the subtraction) = 100.0 – 10.00 = 90.0 15 II. Scientific Notation A. Using Scientific Notation In science, we have the opportunity to work with both extremely large and extremely small numbers. However, numbers like this are often extremely cumbersome to write: 602,000,000,000,000,000,000,000 0.000000000000000000000000000911 Scientific notation is a way of writing numbers that is especially useful for very large or very small numbers. In the examples above, the information about the number’s specific value is contained in the 602 and the 911, while the rest of the zeros only indicate the placement of the decimal point, and do not count as significant figures (they are either trailing zeroes or leading zeroes, respectively). In scientific notation, these numbers can be written more compactly as: 602,000,000,000,000,000,000,000 6.021023 0.000000000000000000000000000911 9.1110-28 Scientific notation is based on the idea that multiplication or division by 10 does not change the value of the number, only the position of the decimal point: 1. Multiplying a number by 10 moves the decimal point one place to the right every time you multiply by 10: 7.61 10 = 76.1 2. 0.0053 10 = 0.053 Dividing a number by 10 moves the decimal point one place to the left every time you divide by 10: 85.21 8.521 10 3. 4.382 10 10 = 438.2 0.00633 0.000633 10 9163 9.163 10 10 10 Multiplying by 10 several times can be indicated by using positive exponents on the 10: 6.132 10 10 10 = 6.132103 4. Dividing by 10 several times can be indicated by using negative exponents on the 10: 4.554 4.554 10 2 10 10 In scientific notation, we indicate the specific digit values with a number between 1 and 10, followed by a 10 raised to some exponent which indicates how many times the specific digit values must be multiplied or divided by 10 to obtain the desired decimal number: 16 (1-10)10exponent (The meaning of several exponents is listed in Table 1.) For example, the number 602,000,000,000,000,000,000,000 is written as 6.021023, since multiplying 6.02 by 10 twentythree times produces the original number. Likewise, 0.000000000000000000000000000911 is written 9.1110-28, since 9.11 must be divided by 10 twenty-eight times to produce the original number. Notice that 52.47108 is not in scientific notation, because the digit information 52.47 is not between 1 and 10. It must be changed to 5.247109 to be in proper scientific notation. Table 1. Exponents in Scientific Notation 106 105 104 103 102 101 100 10-1 10-2 10-3 10-4 = = = = = = = = = = = (10)(10)(10)(10)(10)(10) (10)(10)(10)(10)(10) (10)(10)(10)(10) (10)(10)(10) (10)(10) (10) (1) 101 = (0.1) 101 101 = (0.1)(0.1) 101 101 101 = (0.1)(0.1)(0.1) 101 101 101 101 = (0.1)(0.1)(0.1)(0.1) = = = = = = = = = = = 1,000,000 100,000 10,000 1,000 100 10 1 0.1 0.01 0.001 0.0001 Thus, to write a number in scientific notation, take the original number and move its decimal point either left or right to produce a number between 1 and 10. Then use a power of 10, either + or -, to indicate how many places the decimal point was moved. If the decimal point was moved to the right, make the exponent (-); if the decimal point was moved to the left, make the exponent (+). If there is already a power of 10, moving the decimal to the right makes the exponent more negative by the number of decimal places moved, while moving the decimal to the left makes the exponent more positive. The following examples illustrate these rules. Original No. 0.00512 647 583.71106 0.000768810-3 0.00531102 Scientific No. 5.1210-3 6.47102 5.8371108 7.68810-7 5.3110-1 Comments Decimal point moved three places to right. Decimal point moved two places to left. Decimal to left, 6 becomes more positive by 2. Decimal to left, -3 becomes more negative by 4. Decimal to right, 2 becomes more negative by 3. Negative exponents are sometimes used on units or symbols as well as on numbers. Just as 1/10 can be written as 10-1, likewise 1/x can be written as x-1, or 1/x3 as x-3. In the same way, g/mL can be written as g·mL-1 or cm/sec2 as cm·sec-2. Numbers like 102 or 10-4 should be considered as abbreviations for 1102 or 110-4. Also note that a number such as 5.73 can be written as 5.73100, since 100 equals 1. 17 B. Arithmetic Using Scientific Notation Significant Digits. One advantage of scientific notation is that all significant digit information is contained in the 1-10 portion of the number. For example, 6.510-17 contains two significant digits, and 4.8113107 contains five significant digits. Even the ambiguous cases can be clarified, such as the number 5000: to indicate one significant digit, you would write 5103, to indicate two significant digits you would write 5.0103, to indicate three significant digits you would write 5.00103, and so on. Multiplication. All arithmetical operations on numbers written in scientific notation are performed in two separate parts, one on the 1-10 segments and one on the exponents of 10. Note: Calculators that do exponential arithmetic perform both parts simultaneously. To multiply two exponential numbers, first multiply the two 1-10 parts. Then obtain the new power of 10 by adding the two original powers of 10. (3.7102)(2.21103) = 8.2105 (1.3310-12)(6.412104) = 8.5310-8 (5.7110-6)(4.2310-7) = 2.4210-12 The last example illustrates a common error: it is easy to look at the exponents -6 and -7, and expect a final exponent of -13, forgetting that (5.71)(4.23) = 24.2, not 2.42. Thus 24.210-13 must be written in correct scientific notation as 2.4210-12. Division. To divide one exponential number by another, first divide the two 1-10 parts in the proper order. Then obtain the new power of 10 by subtracting the two original powers of 10 (exponents) in the order top minus bottom. 7.143 10 8 2.80 10 83 2.80 10 5 2.55 10 3 5.6 10 9 1.4 10 9( 3) 1.4 1012 3 3.89 10 2.971 10 4 0.678 10 4( 7 ) 0.678 10 3 6.78 10 2 7 4.38 10 Addition/Subtraction. To add or subtract two or more exponential numbers, each number must first be written so that all have the same power of 10 (usually the most positive exponent), even though some may not be in exponential form. Then the parts between 1-10 are added or subtracted, and the new power of 10 is just the power of 10 to which all numbers were changed. Answers are rounded to proper number of significant digits as indicated by vertical dotted line. 18 6.132103 + 2.88104 1.6610-3 – 8.3410-4 0.6132104 2.88104 3.4932104 3.49104 1.6610-3 0.83410-3 0.82610-3 0.8310-3 ═ 8.310-4 Raising to a Power. To raise an exponential number to a power, first raise the 1-10 part to that power. Then to get the new power of 10, multiply the original exponent of 10 by the power of which the number is being raised. (1.6105)3 = (1.6)310(53) = 4.11015 (5.7210-9)4 = (5.72)410(-9)(4) = 1070.4910-36 = 1.0710-33 Extracting a Root. To take the root of an exponential number, first take that root of the 1-10 part. Then to get the new exponent of 10, divide the original exponent by the root being taken. If the original exponent is not initially exactly divisible by the root, first re-write the entire exponential number, moving the decimal to the right enough places so that the new exponent of 10 is exactly divisible by the desired root. 6.32 108 6.32 10 (8 / 2) 2.5110 4 3 1.92 1013 3 19.2 1012 3 19.2 10(12 / 3) 2.68 10 4 Scientific Notation on Calculators. Most calculators that do any scientific or higherlevel math functions allow a number to be entered in scientific notation. Texas Instrument calculators usually have a button marked “EE” [or on some newer calculators as “10x”], which allows the number 6.021023 to be entered as “6.02” + “EE” + “23” (on the screen, it will look like 6.02E23). Casio and Sharp calculators use a button marked “EXP” to accomplish the same thing; HP calculators use a button marked “E.” [Note: Do not confuse those buttons with the “ex”, “exp(x)”, or “10x” buttons, which are logarithmic functions, and are usually paired on the same button with the “log” and “ln” functions.] 19 III. Units of Measure A. Base SI Units. The system of units which is used in the sciences is the modern form of the metric system, the International System of Units, or the SI system (from the French Le Système International d’Unités), as defined by the Eleventh General Conference on Weights and Measures in 1960, and adopted by the National Bureau of Standards in 1964. There are seven base units in the SI system, and all other units of measure are derived from these seven: Physical Quantity Unit Symbol Length Mass Time Temperature Amount of substance Electric current Luminous intensity meter kilogram second kelvin mole ampere candela m kg s K mol A cd Base units are defined ideally by some physically measurable and reproducible property. For example, the meter (m), the SI base unit of length, is defined as “the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 of a second.”2 Only one SI unit is based on a single prototype object or standard: the kilogram “is equal to the mass of the international prototype of the kilogram”2 which is stored by the International Bureau of Weights and Measures in a vault in France. The collection of base units in a measurement system is the smallest set of units from which all other measurements can be defined and expressed in terms of algebraic combinations. B. Decimal Multipliers (Metric Prefixes). When dealing with inconveniently large or small numbers, decimal multipliers are often used to adjust the order of magnitude (power of 10) of the measurement. For instance, the prefix “kilo” (abbreviated as “k”) means 103 or 1000, so a kilometer (km) is 1000 meters. Similarly, the prefix “milli” (m) means 10-3 or 0.001, so a millimeter (mm) is 0.001 meters. For example: 12300 meters = 12.3 km 0.00123 m = 1.23 mm Table 1 lists the decimal multipliers that are used in the SI system; the multipliers marked with an arrow () are the most commonly used prefixes, and should be memorized. 2 Ambler Thompson and Barry N. Taylor, Guide for the Use of the International System of Units (SI), NIST Special Publication 811 2008 Edition, National Institute of Standards and Technology, Gaithersburg, MD 20899, March 2008, p. 39. [See http://physics.nist.gov/cuu/pdf/sp811.pdf and http://physics.nist.gov/cuu/Units/ for more information.] 20 Table 1. Decimal Multiplies in the SI System. Fraction Prefix Symbol Example Scale 1024 yotta Y volume of earth ~1 YL 1021 zetta Z radius of Milky Way ~1 Zm 18 10 exa E age of universe ~0.4 Es 1015 peta P 1 light-year ~9.5 Pm 12 10 tera T distance from sun to Jupiter ~0.8 Tm 9 9 10 giga G 1 Gm = 10 m 1 light-second ~0.3 Gm 6 6 mega M 1 Mm = 10 m 1 Ms ~11.6 days 10 3 kilo k 1 kg = 1000 g 10 2 10 hecto h 1 hg = 100 g 101 deka da 1 dam = 10 m 0 10 — — -1 10 deci d 1 dm = 0.1 m -2 centi c 1 cm = 0.01 m 10 -3 milli m 1 mg = 0.001 g 10 -6 micro μ 1 μm = 10-6 m 1 µL ~a very tiny drop of water 10 -9 nano n 1 ns = 10-9 s radius of Cl atom ~0.1 nm 10 -12 -12 10 pico p 1 pm = 10 m mass of bacterial cell ~1 pg -15 10 femto f radius of a proton ~1 fm -18 10 atto a time for light to cross an atom ~1 as 10-21 zepto z 1 zmol ~600 atoms -24 10 yocto y 1.7 yg ~mass of a proton or neutron (Prefixes marked with should be memorized) C. Derived Units . Derived units are obtained from mathematical combinations of the base units. For instance, area is given by the formula Area = length width Since both length and width are measured in meters in the SI system, the SI derived unit for area is meter2 (square meters, or m2). The velocity of an object is the distance covered per unit of time (velocity = distance / time) so in the SI system velocity has units of meters/second (meters per second, m/s, or m·s-1). Acceleration is the rate of change of velocity per unit of time (acceleration = change in velocity / time), so acceleration has units of meters/second2 (meters per second per second, m/s2, or m·s-2). Some derived SI units are given special names. For example, the SI unit for force (force = mass × acceleration) is the Newton, which is defined as 1N ≡ 1 kg·m·s-2, and the SI unit for energy (energy = force × distance) is the joule (J), which is defined as 1J ≡ 1 N·m ≡ 1 kg·m2·s-2. 21 Table 2. Some SI Derived Units. Quantity Definition Units Area m2 Length width Volume Length width height m3 Density Mass / volume kg/m3, g/cm3, g/mL Speed Distance / time m s-1 Acceleration Change in speed / time m s-2 Frequency Event / time s-1 (hertz, Hz) kg m s-2 (newton, N) Force Mass acceleration Pressure Force / area kg m-1 s-2 (pascal, Pa) kg m2 s-2 (joule, J) Energy Force distance Only derived units that are derived from the SI base units are considered to be SI units. For instance, m3 is the SI derived unit for volume; however, the liter (L), defined as 1 dm3 (1 dm ≡ 10-1 m), while not considered to be an SI unit, is approved for use by SI guidelines. (Because of this definition, 1 mL = 1 cm3 = 1 cc.) D. Conversions Between Different Units . Some measurements can be reported in a variety of possible units: for instance, length can be reported in meters, kilometers, centimeters, millimeters, feet, inches, yards, statute miles, nautical miles, fathoms, furlongs, leagues, angstroms, chains, or light years. Appendix I lists a number of relationships which can be used to convert numbers from one unit into another unit. The next section of the manual discussion the use of conversion factors and the manipulation of units in more detail. 22 IV. Dimensional Analysis A. Ratioed Properties and Dimensional Analysis. Every number that you measure in the laboratory, and most of the numbers you calculate, will have not only a numerical value but also a set of units associated with it, such as 5.12 grams (g) or 31.5 milliliters (mL). These units can be manipulated (in fact, must be manipulated) in calculations just like numbers. For example: 6 cm 2 cm 4 cm 8.0 ft 2.0 ft 16 ft 2 24 ml 6.0 (no units) 4.0 ml mL/mL can also be written as mL·mL-1 = mL0 = 1 (no units) Some units cannot be combined further, but do represent valid physical quantities, such as density or energy: 6g 3 g/mL or 3 g mL-1 2 ml 5.0 ft 3.0 lb 15 ft lb Some units may seem strange, such as 0.00165 s-1 or 0.00165 /s (read “per seconds” or “reciprocal seconds”). The unit of time-1 is a unit of frequency, not time. Dimensional analysis is a method of analyzing the set-up of a problem by manipulating the units in the same way you plan to manipulate the numbers. If your final units are correct, your problem has probably been set up correctly. Likewise, if your final units are incorrect, or you obtain nonsense units, your problem is not set up correctly. Many of the properties that are dealt with in this class involve ratios — for instance, the density of an object is the ratio of its mass to its volume (density = mass / volume), the speed of an object is the ratio of the distance traveled to the time (rate=distance/time), and so on. Even quantities such a molar mass can be considered to be “ratioed properties” (in this case the ratio of the mass of an object to the amount of the substance measured in moles). Most computations involving ratioed properties can be performed by paying attention to the units in the ratio. Keys to using dimensional analysis: 1. A ratioed property relates two properties that define that ratioed property. (For example, mass and volume are related by density.) 2. This relationship allows one defining property to be determined from the other. (The mass of a sample can be determined from the density if the volume is known.) 3. Conversion is accomplished by multiplying by the ratioed property or its reciprocal expressed as fractions, as demonstrated in the following examples. 23 Suppose you want to convert 5.0 pounds (lb) to kilograms (kg) and you know that 2.2 pound = 1 kg. You are unsure, however, whether to divide 5.0 by 2.2, 2.2 by 5.0, or multiply 2.2 by 5.0. The units tell you the correct choice. You know your final answer should be in units of kg. So try the following: 2.2 lb 11 lb 2 /kg (5.0 lb) 1 kg incorrect: lb2/kg is not a unit of mass 2.2 lb 1 -1 0.44 /kg or 0.44 kg 1 kg 5.0 lb incorrect: kg-1 is not a unit of mass 1 kg (5.0 lb) 2.3 kg 2.2 lb correct: kg is the desired unit of mass As another example: How many cm3 (cubic centimeters) are there in 2 m3 (cubic meters)? You know that 1 m = 100 cm, and m3 = mmm. So you try 100 cm 2 (2 m 3 ) 200 m cm 1 m The units are not correct, since m in the denominator only cancels one of the m’s in m3. To cancel out all of the m’s in m3, the conversion factor needs to be cubed: 3 100 cm 100 cm 100 cm 3 100 cm 3 3 (2 m 3 ) 2,000,000 cm 2,000,000 cm or (2 m ) 1m 1 m 1 m 1 m (Note that everything in the parentheses in the last step must be cubed; that is, the conversion factor should be read as 13 m3 = 1003 cm3.) This approach can facilitate multi-step conversions, such as converting 58 cm3 to gallons: 1 cm3 = 1 mL 1 L = 1000 mL 1 qt = 0.946 L 1 gal = 4 qt 1 mL 1 L 1 qt 1 gal 0.015 gal (58 cm 3 ) 3 1 cm 1000 mL 0.946 L 4 qt This approach is also helpful when dealing with conversions that involves numbers where there is a unit in the numerator and the denominator (such as miles per hour or grams per milliliter). For example, if you want to convert 55.0 miles per hour (mi/hr) into units of meters per second (m/s), write the number as a ratio: 24 55.0 mi 1 hr and look at where each conversion factor needs to be placed to cancel out the appropriate unit: 1 mi = 5280 ft 1 m = 3.281 ft 1 hr = 60 min 1 min = 60 s 55.0 mi 5280 ft 1m 1 hr 1 min 290400 m or 24.6 m/s 1 hr 1 mi 3.281 ft 60 min 60 s 11811.6 s 25 V. Accuracy and Precision; Standard Deviations A. Accuracy and Precision. Whenever possible, a measurement must be performed more than once in order to improve the confidence with which the result is reported. The precision of a series of measurements is a measure of how close all of the reported numbers are to each other, while the accuracy of the measurements is a measure of how close they are to the actual value. For example, consider the archery target in Figure 1 below. In Figure 1a, the arrows have struck the target all over the place: the arrows are not clustered together anywhere, and there is only one in the center. The results are inaccurate and imprecise. (a) (b) (c) Figure 1. Precision and accuracy on the archery range. In Figure 1b, the archer’s aim has improved: the arrows are clustered together, but they are not at the center of the target. The results are precise (because they are all close together), but inaccurate (because they are not in the center). Finally, in Figure 1c, the arrows are all clustered together in the center of the target. The results are both accurate and precise. The results in Figure 1a are due to random error — there is no pattern to how the arrows have hit the target. In measurements, random errors often average out in repeated trials. In Figure 1b, there is a systematic error — the aim is off in one direction. Systematic errors do not average out in repeated trials, because the same error is being made every time. (For example, if a balance is not calibrated properly, it may report a value which is either too high or too low.) B. Standard Deviations. Standard deviations are a measure of how data are distributed around a mean (average) value. A small standard deviation indicates that the measurements are close together (high precision), and a large standard deviation indicates that the measurements are spread out (low precision). 26 Typically, for a series of measurements, the sample standard deviation, s, is used. This value is calculated from the following formula: s 2 1 N xi x N 1 i 1 where N xi = the number of measurements = a particular measured value x = average (mean) = sum of the variables The steps in taking a standard deviation are summarized below (these step numbers are illustrated in the examples on the next page): 1. For a series of N measurements, compute the average ( x ). 2. Take the difference between each data point and the average ( xi x ) 3. Square the difference. 4. Add up the squares of the differences. 5. Divide the sum of the square by N-1. 6. Take the square root of that value. The standard deviation is a measure of how closely a set of measurements agree with each other. A low standard deviation means that the measurements are all fairly close together, and the measurement is therefore precise. A high standard deviation means that the measurements are more spread out, and the measurement is imprecise. This does not necessarily say anything about the accuracy of the measurement, however: a systematic error, such as an improperly calibrated balance, can result in data which are precise, but inaccurate. Many scientific calculators have statistical functions which will allow them to compute standard deviations; it is necessary first to make sure that it is the sample standard deviation that is being calculated, and not some other kind of standard deviation. In Microsoft Excel, a series of values can be reported in a column, and the function =STDEV(array) can be used to calculate the sample standard deviation. 27 Example 1: In the following example, a series of five measurements is summarized in the first column: 12.12, 12.16, 12.14, 12.15, and 12.18; the average of these values is 12.15. The columns labeled 1 through 6 illustrate the steps in calculating a standard deviation that are described on the previous page. The standard deviation is calculated to be 0.022, and the value that is reported for that measurement is 12.15 ± 0.02. Example 1: Step: 1 2 3 4 5 6 2 2 ` x xi x i - ` x (x i - ` x ) (x i - ` x ) /(N-1) √[/(N-1)] 12.12 12.15 -0.03 0.0009 0.0020 0.0005 0.022 12.16 12.15 0.01 0.0001 12.14 12.15 -0.01 0.0001 12.15 12.15 0.00 0.0000 Reported value: 12.15 ± 0.02 12.18 12.15 0.03 0.0009 Example 2: In the following example, a series of five measurements is summarized in the first column: 12.00, 12.85, 12.50, 13.05, and 10.35; the average of these values is 12.15. Note that this is the same average as in Example 1, but the individual measurements are much more spread out. The standard deviation is calculated to be 1.08, which is a much larger value than in Example 1; the value that is reported for the measurement is 12.15 ± 1.08. Example 2: Step: 1 2 3 4 5 6 2 2 ` x x i - ` x (x i - ` x ) (x i - ` x ) /(N-1) √[/(N-1)] xi 12.00 12.15 -0.15 0.0225 4.6850 1.1713 1.082 12.85 12.15 0.70 0.4900 12.50 12.15 0.35 0.1225 13.05 12.15 0.90 0.8100 Reported value: 12.15 ± 1.08 10.35 12.15 -1.80 3.2400 28 VI. Properties A. Chemical and Physical Properties. Chemists use properties to describe, organize and understand matter. Properties can be classified in a number of different ways. One way to classify properties is as chemical or physical properties. A physical property can be measured without changing the composition of the sample. The sample is the same substance before and after the property is determined. Some examples of physical properties include: Mass and volume. Malleability — the ability of an object to be deformed by hammering without shattering. Most metals are malleable — they can be hammered into thin sheets without changing any of their chemical properties. Brittleness — a physical property that is the opposite of malleability. Table salt is brittle — if pounded, it shatters; however, the smaller particles that are formed are still salt, as can be determined by tasting or by chemical analysis. Melting point — the temperature at which a solid becomes a liquid. Boiling point — the temperature at which a liquid becomes a gas. A chemical property is one which is observed when a substance’s composition is changed, by undergoing a chemical reaction. When a chemical change occurs, a substance becomes a different substance. For example, combustibility, the ability of a material to burn, can only be determined by trying to ignite the material — when paper, natural gas, or gasoline burn, they are no longer paper, natural gas, or gasoline. Experimentation shows that they are converted primarily to water and carbon dioxide. Sometimes, it is more difficult to distinguish whether or not a change is physical or chemical. Consider the conversion of water to steam. On first inspection, we might conclude that since the properties of water and steam are so different, that they are different substances, and that a chemical reaction has occurred. In reality, the difference is not in the samples, but in the conditions under which the properties are being measured. If the steam is cooled to room temperature, it will return to the liquid state, and will be indistinguishable from the original water. Thus, this is a physical change, not a chemical one. Another example is the change that occurs when table salt is dissolved in water. Since the solid form of salt is no longer present, you might conclude that a chemical change has occurred, but on closer inspection, we can see that other properties of salt, such as its taste, are still present. Again, the difference is in the conditions rather than in the samples. In one case, we are observing the properties of pure salt (in the absence of water). In the second case, we are observing the properties of salt in presence of a large excess of water. If we treat the salt solution by evaporating away the water, so that both samples are present under the same dry conditions, we will then observe that both samples have all of the same properties and are the same substance. The trick is to make sure that we observe the samples under the same conditions. Now consider the combustion of gasoline. If we burn gasoline, and cool the products to room temperature, there will be a liquid and a gas. 29 Examination of the liquid demonstrates that it is very different from gasoline. For example, it does not have the characteristic smell of gasoline, and it evaporates at a substantially lower rate. In addition, neither the liquid nor the gaseous products burns (as does the gasoline) or supports combustion (as does the oxygen that reacts with the gasoline during combustion). Clearly these products are different substances than the starting materials. This is a chemical change, in which gasoline and oxygen have been chemical recombined to form carbon dioxide and water. Sometimes observing samples under similar conditions is not sufficient to distinguish between physical and chemical processes. The gas that is responsible for the brown color of smog is nitrogen dioxide (NO2). At higher pressures, NO2 is converted to colorless dinitrogen tetroxide (N2O4). If the pressure is lowered again, it is converted back to NO2. A similar effect can be observed if it is heated and cooled substantially. In this case, the color change is a good indicator that a chemical reaction is occurring. Change in color is often, but not always, a useful indicator of a chemical process. B. Extensive Versus Intensive Properties Properties can also be divided into extensive and intensive properties. Extensive properties vary with the amount of the sample. Mass and volume are extensive properties: if the amount of a material is doubled, the mass and volume are doubled. Extensive properties are very useful to chemists for determining how much of a substance or material is present. Measuring how much of something we have is referred to as quantitative analysis. Intensive properties do not vary with the amount of the sample (they are independent of the sample size). The chemical property of combustion is an intensive property: gasoline of any amount will burn if ignited in the presence of oxygen (although the amount of heat that is generated will be different depending on the amount of gasoline that is burned). Melting points and boiling points are also intensive properties: water freezes at 0ºC and boils at 100ºC whether you have a teacup full of water or a swimming pool full of water. Since intensive properties do not change with sample size, they can be used to identify what a sample is made of. Identifying what substance or substances make up a sample is known as qualitative analysis. Density (d) is defined as the ratio of an object’s mass (m) to its volume (V): density mass volume d m V Mass and volume are extensive properties, but for a pure substance, the density of the substance is an intensive property, which is independent of the sample size. In this case, both mass and volume change with the size of the sample, but they are both changing by a proportional amount because this is a ratioed property. (It is important to remember that even though the density of a sample is not affected by sample size, it is affected by the temperature of the sample: many substances expand when heated, which changes the V term in the equation. When reporting a 30 density measurement, it is important to report the temperature at which the measurement was made.) 31 Experiments Got Moles? 32 The first essential in chemistry is that thou shouldst perform practical work and conduct experiments, for he who performs not practical work nor makes experiments will never attain the least degree of mastery. Jabir ibn Hayyan (Geber) (721-815)