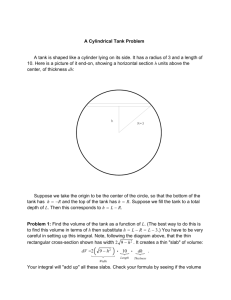
第4章 処理特性 - modelEAU
advertisement

Appendix 1 Definition and explanation of term 279 Appendix1 Definition and explanation of term The explanation of the important key word judged that describing clearly and the explanation of the definition are necessary among terms used in this report is individually added. The one thought that the explanation of the one used in a meaning different from a usage (a) general and the limited meaning and the (b) supplementation was usefulness on the business was published though the one published in "Drainage glossary 2000 editions (corporation Japan Sewage Works Association)" was basically omitted though it was a special term. As for the one judged that the generality of the usage was not enough among these, the effect that was the term that fixed "*" to finding, and was used in the peculiarity by this report was clear. Moreover, after the explanation, this pertinent page of [shi] exhibit materials was fixed to the tail about the one that the explanation is in the report. ≪ alphabet ≫ ASM Abbreviation of "Activated Sludge Model (activated sludge model)". In general, it is used generically of the activated sludge model of IWA. This report is used generically of ASM series (ASM1-3). (→ pp. 8, 10) CODCr Oxygen demand with dichromate potassium. The consumption of the dichromate potassium when the oxide in the sample is oxidized by using the dichromate potassium as an oxidant by one of the water quality parameters that express the amount of the organism in water is measured, and this is converted into the oxygen consumption. Because the oxidizing power of the dichromate potassium is strong, the catching rate of the organism is higher than that of CODMn. It is used from obtaining measurements near the absolute magnitude of the organism as a carbon-estimating parameter by the activated sludge model of IWA. (→ pp. 30, 323) CODMn Oxygen demand with overmanganese potassium. The consumption of potassium permanganate when potassium permanganate is made an oxidant by one of the water quality parameters that express the amount of the organism in water and the oxide in the sample is 280 Appendix 1 Definition and explanation of term oxidized is measured, and this is converted into the oxygen consumption. The catching rate of the organism is smaller than that of CODCr, and it is more unsuitable though it is an index COD generally used in our country as the revenue and expenditure index. Because a part of the organism such as the amino-acid, organic acids, and the fatty acids biologically used easily is hardly oxidized, it is usual to indicate the small value from BOD5 in the domestic wastewater. (→ pp. 55, 324) Appendix 1 Definition and explanation of term 281 Expression of Monod In the empirical equation that expresses the substrate concentration dependency of the proliferation rate of the microorganism proposed by Monod, it is the most general as the mathematical expression of the proliferation rate. It is characterized by the maximum specific growth rate and two constants of saturation factor, and the speed decreasing rate to the maximum specific growth rate is calculated by the magnitude correlation of the substrate concentration and saturation factor. To come extremely ..greatly.. ..the zero-order reactions.. more extreme than saturation factor the substrate concentration with the small, it approximates as an expression of first order reaction. Both are essentially different though it is the same type as the expression of Michaelis-Menten that is mathematically the theoretical formula of the enzyme reaction rate. (→ pp. 21) OUR Abbreviation of "Oxygen Uptake Rate (oxygen utilization speed)". The one that use speed of oxygen by living being in samples such as inflow drainage and activated sludge was measured. Measuring changing DO, and calculating again as a decline in oxygen for each amount of the unit solid for each unit volume are general. In the field of the activated sludge model, it is used as one of the experimental techniques to presume parameter value of the amount of the organism element and the model in influent. (→ pp. 116) PHA Abbreviation of "Polyhydroxyalkanoate (polyhydroxyalkanoate)". Generically of two or more compounds, PHB (polybeta-hydroxybutyric acid) is typical. It is synthesized by a lot of microorganisms as a store organism, and it is stored to intracellular. It plays an important role as a store organism by phosphorus accumulation living being (PAO) in the biological phosphorus removal process. It is not identical with PHA that is contained other store organisms such as glycogens in this XPHA defined by ASM2d is modeling this, and is measured by chemical analysis. (→ pp. 32) -t Width of time interval of numerical calculation. It becomes base unit when the amount of the time variance of each variable is calculated by the simulation. Computing time becomes long a small setting of this though it is desirable to set a small value enough to the variable ratio of a function of change of variable value. There is a simulator that uses the computational algorithm to adjust this by the automatic operation though it is basically a numerical value that the user should set, too. (→ pp. 157) 282 Appendix 1 Definition and explanation of term It is ≪ or ..doing.. ≫. Hydrolysis Activated model Stoichiometry Complete Generic name of reaction that unites with water organic/inorganic compound and is converted into another compound. When the organism under drain is converted, the key role is borne as a reaction from which high molecular compound that cannot take the bacillus directly into intracellular in the biological waste water treatment process is made a low molecular weight. It is thought that the enzyme reaction caused on the cell surface is a subject. It is known to progress from reluctance to the conceit on a wide condition. The finding concerning a kinetics characteristic is insufficient though it is thought that the speed decreases under the anoxic condition reluctance/Though it is defined as a process that XS converts into SF in ASM2d, the hydrolysis process will bear a part of a lot of phenomena involved in living being's such as the use of the intracellular store organism and preying done by the protozoan autolysis for the model structure (concept of autolysis-reproduction) that XS generated in the autolysis process of the living being similarly contributes to the consumption of oxygen and the nitrate through hydrolysis, too, and it is different from a pure hydrolysis reaction. Because the concept of endoecism breath is adopted in ASM3 and the store and the their use of the organism were modeled separately, the contribution of the hydrolysis process is decreased. (→ pp. 32) sludge Biological response model by whom internal structure of various phenomena in activated sludge mainly composed of biological response is mathematically described. The simulation of the activated sludge process becomes possible incidental models such as the sedimentation ponds and the diffused air because it models the entire combination activated sludge process this. On the business, the activated sludge process model including the incidental model might be only called an activated sludge model. (→ pp. 6) It uses it in this report though the entire study that handles a quantitative character and the relation of the compound is indicated to the wide sense by the wording in the narrow sense that means a relative relation of the variation of the compound by the process of the reaction. It is an important corner of the content of the description of ASM, and it is displayed by the matrix type. Refer to "Amount of theory coefficient". (→ pp. 14) mixing Reactor where all materials in tank exist uniformly. The material Appendix 1 Definition and explanation of term tank 283 that flows in is always uniformly instantaneously distributed to the entire tank, and the composition of the discharge becomes in the tank and identical. It is difficult to actually achieve such a state, and has an intermediate characteristic of the complete mixing tank and the plug flow tank by a reactive tank of the active sludge process. It is used as base unit that composes a reactive tank in the activated sludge process model do not to have to consider the spatial gradient of the element in the tank and to excel in mathematical handling. (→ pp. 139) 284 Appendix 1 Definition and explanation of term Sensitivity analysis It is indicated to examine the response of the output to the model to the logical input value. When the data collection, the calibration, and the simulation result are evaluated by examining the influence that parameter value, the facilities condition, the inflow condition, and the operating condition, etc. in the model give the simulation result, useful information can be obtained when the business of ASM is used. (→ pp. 157, 367) Calibration Work to adjust parameter value etc. of model and to improve accuracy of expectation. The entire work to improve the reproducibility of the processing results of the object facilities including the facilities condition, the influent quality, and the adjustment of the input data of the operating condition etc. and the verification of the adjustment result is located with the calibration in this report though the optimization of parameter value in the model is typical. (→ pp. 146) Ultimate BOD BOD value when BOD examination is executed for a long term, and state that all organisms of possible biodegradation in sample (The amount of the organism of the living being is included) are oxidized is assumed. It might be written as BODu or UBOD, etc.In general, the model assumed to be progress of the oxidation of the organism to remaining BOD by first order reaction for the BOD value in which the same sample was measured at two or more periods is applied and calculated. When the organism element of influent is drawn in the amount as an index that reflects the gross weight of the organism that can be biologically resolved, it is possible to use it when the activated sludge model is used. However, because part remains finally as an inert organism and doesn't contribute to BOD measurements, it is necessary to correct this though it was used for biosynthesis among organisms of a possible biodegradation that exists in the sample while culturing it. (→ pp. 119) Glycogen It is wide in the organism and exists by a kind of the polysaccharide as an energy storage material. When do the metabolism of PAO that bears the biological phosphorus removal, it has the key role as a reducing power source of supply for the PHA synthesis under the anoxic in waste water treatment (However, the accumulated dose of the glycogen ..behavior of PAO.. doesn't usually become a phase rule [suru] condition easily). In addition, glycogen accumulation living being (GAO) that makes not the polyphosphate but the glycogen an energy source and stores the organism under the anoxic is one factor of the deterioration of the biological phosphorus removal. The metabolism of the glycogen by PAO is not modeled in ASM2d, and the glycogen is not defined as an independent element (It is included in XPHA as an amount of the organism). Appendix 1 Definition and explanation of term 285 (→ pp. 43, 50) 286 Appendix 1 Definition and explanation of term Glycogen accumulation being living The living being that has the ability to convert calles it generically to ..organic substrate such as acetates.. intracellular as an energy source and a reducing power source of supply of an intracellular glycogen under the anoxic the reserve substance such as uptakes and PHA. It is abbreviated with "GAO(Glycogen Accumulating Organisms)". In aerobic condition, regeneration and the proliferation of the glycogen using intracellular PHA are done as well as PAO. The polyphosphate is made an energy source and the behavior seen in PAO like Rin's discharge and uptake, etc. doesn't accompany the place because it doesn't require it. The phosphorus removal deteriorates when it is in the relation that competes with PAO in the biological phosphorus removal process, and the ratio of GAO increases. The contribution of GAO can indirectly be expressed as an element by parameter value of XPAO because it is included in XPAO though this living being is not modeled in ASM2d. (→ pp. 44, 50, 176) Work to confirm the predictive accuracy of the model is said. It is important to use, to verify a different data set because in the calibration of the model, there is danger of falling into the result of specializing in the data used there, and to confirm the generality of the calibration result. (→ pp. 177) Verification ..doing.. ≫ ≪ Intracellular material Oxygen speed store Generic name of compound stored to intracellular. The polyphosphate is typical as PHA, the glycogen, and inorganic compound as organic compound. The intracellular store material is properly resolved, and used , saying that the energy source and the carbon source. In the biological phosphorus removal process, Rin is removed by being exhausted outside the faction as an excess sludge with PAO shows feature behavior through PHA, the glycogen, and the polyphosphate, and the polyphosphate accumulated in intracellular in a high density finally. To model this, two intracellular store materials named XPHA and XPP are defined in ASM2d. Moreover, it is assumed that heterotrophs XH other than PAO proliferate through the store, and has been changed the approach that expresses the use of the organism greatly in ASM3. (→ pp. 46) utilization Refer to "OUR". Appendix 1 Definition and explanation of term Autolysis 287 Generic name of phenomenon of weight and revitalization of bacillus decreasing. There are the one by an inner factor and the one by the external factor, and consumption, the resolution, and the latter of an intracellular compound like the reserve substance etc. include preying by the protozoan and bacteriolysis by the chemical substance and the pH, etc. in the former. It is more important to express "Endoecism breath" phenomenon observed from the accurate expression of this from a microbiology aspect when the activated sludge process is modeled as a phenomenon, adopts "Concept of autolysis-reproduction" as an approach for that in ASM1-2d, and "Concept of endoecism breath" is adopted in ASM3. In ASM2d, the autolysis process is defined respectively about intracellular store material XPHA and XPP of another and PAO from which an independent autolysis process is defined in three kinds of living being elements respectively. The process where the intracellular store material is used is separately defined in XPAO though the autolysis process of XH includes all phenomena of the biomass's like preying by the protozoan and the use of the intracellular store material, etc. decreasing. (→ pp. 32) Concept of It is adopted with ASM1-2d by one of the modeling methodologies of autolysis-reproducti "Endoecism breath" phenomenon. Living being's autolysis generates the organic substrate and the modeling is done by assumption that on oxygen (It is a nitrate in the anoxic condition) is consumed when accompanying (Part becomes an inert organism) and this are used again. As for "Autolysis" process of the living being, when it is defined as a mere transformation process from the living being to XS and XI, and caused XS is used again through the hydrolysis process, oxygen and the first nitrate are consumed in ASM2d. The point that the loop of the organism is formed in a series of process from hydrolysis to autolysis is a big feature. Because a part of the amount of autolysis is used to proliferate again about XH and XPAO, it is necessary to use a value that is bigger than the endoecism respiratory rate experimentally observed for the autolysis rate constant (bH and bPAO, etc.). Moreover, because a part of the organism of the autolysis origin is converted into XI when self-resolved again after it proliferates, yield fXI of XI is set smaller than usual endoecism breath models. On the other hand, because XS after autolysis is not used for XAUT about XAUT, autolysis rate constant (bAUT ) becomes the decline and equal the living being (revitalization) experimentally observed. "Concept of endoecism breath" is adopted in ASM3, and a big change point of the model concerned (Refer to "Endoecism breath"). (→ pp. 32, 35) Scenario * Generic name of condition setting it as examination object by simulation. For instance, when there is a target of the simulation "Processing status when the amount of the oxygen supply is decreased more than the current state is expected" in the given activated sludge process, an individual calculation condition of 288 Appendix 1 Definition and explanation of term decreasing the amount of the oxygen supply is considered to be one scenario respectively (The usage of the former is used in this report though the usage that considers one scenario bringing the polarity of the discussion "It is decreased the amount of the oxygen supply" together is done, too). (→ pp. 95) Appendix 1 Definition and explanation of term 289 Simulation It is said to expect and to analyze it on the computer on the basis of the model of the system of the reality. Numerical calculation under the logical input value and parameter value appropriate after the process model that builds this in is constructed on the computer for the activated sludge model is done, and corresponding to work to express and to expect the behavior of the object facilities. (→ pp. 6) Simulator Generic name of application program to execute simulation on computer. It is indispensable to execute the simulation, except when the program can be made by oneself. It is usual to comprise the construction of the process model, condition setting support facility, and the result output function in various methods though the subject of the function is numerical calculation under the logical input value given of the process model. Moreover, there are a lot of one to install various presumption, analysis, and optimization tools (influent quality setting supporting tool, sensitivity analysis tool, and parameter optimization tool, etc.) in a simulator on the market. (→ pp. 9) Heterotroph Generic name of living being that needs organic compound as carbon source for proliferation. Biosynthesis is done by generating energy from taken organic compound by fermentation and breath, and using organic compound taken similarly. The heterotroph of higher-order also influences the clarification etc. of the flocculation and the treated water from the protozoan etc. though the one (heterotrophic bacterium) classified into the bacillus bears the leading part of conversion in the biological waste water treatment process (The denitrificans and PAO are included). "Heterotroph XH" corresponds to these in ASM2d. Though this element chiefly modeled a typical function of the heterotrophic bacterium of the facultative aerobic (The one according to PAO is excluded) on the basis of an average and kinetics and stoichiometry characteristics, the activities of the living being other than the bacillus such as constructional of the model and preying done by the protozoan are partially contained, and it doesn't correspond to a specific bacillus and the living being of the reality (Therefore, the name "Heterotrophic bacterium" is not used). (→ pp. 29) Nitrifying bacteria Generic name of bacillus that bears nitrification. The ammonia is composed in the nitrite and oxidizing ammonium oxidizing bacteria and nitrite are composed of bacterial groups of second classes of oxidizing nitrite-oxidizing bacterium by the ion nitrate. Both majority are autotrophic bacterias of [katayosaga] aerobic (The living being that is the nitrification of the heterotrophy type exists, too), and the proliferation rate and the growth yield are smalls in the carbon fixation because it requires big energy compared with the heterotrophic bacterium. In ASM1-3, the nitrification function is 290 Appendix 1 Definition and explanation of term modeled by living being element XAUT of the kind, and the ammonia does a big simplification of being oxidized (The nitrite is not considered) directly to the ion nitrate there. Organism..amount..ammonium oxidizing bacteria..nitrite-oxidizing bacterium..both..contain..model..growth yield..maximum..specific growth rate..individual..bacillus..one..directly..comparable..attention..neces sary. (→ pp. 29) Appendix 1 Definition and explanation of term 291 State variable Generic name of variable that expresses state of system in model. "Element" is corresponding in ASM in this. Initial condition and the boundary condition are set to each state variable, and the value of each state variable becomes an immediate output in the simulation. (→ pp. 19) Initial condition Generic name of value of each state variable when beginning to calculate at numerical calculation by simulation (initial value). In the simulation using ASM, should it correspond to the initial value of the all component in the calculated all divisions (reactive each tank division, final sedimentation tank, sending back dirt, and internal circulation liquid, etc.), and the user set it. If it is a purpose to obtain the stationary solution after a long term is simulated, the necessity for paying set big attention is a no bur (However, because it influences computing time to reaching regularly by each element, the stationary solution to which the simulation is executed on a certain condition is often given as a initial value). Because initial condition of a floating element has a big influence on the calculation result when simulating it for a short term, an enough discussion is necessary for the set point. (→ pp. 150) Switch function In the reaction rate equation of ASM, the expression paragraph to express the dependency to the density of electron acceptor (SO2 ,SNO3 ) is called generically. An individual process is enabled to be conceited and to express the progression rate in the hypoxia and reluctance each condition by the same rate equation in combining the function of the Monod type and the obstruction type concerning the density of each electron acceptor. (→ pp. 24) Numerical calculation The model expressed by the differential equation etc. is said and the numeric is said under parameter value given of initial condition and the boundary condition solving. Requesting approximation solution from the numeric because it is analytical this impossible to solve it becomes the substance of the simulation ("Solution of the equation" is a value of the state variable (= element) here at all divisions and the total times). As a numerical calculation method though ASM is described as a mathematical simultaneous ordinary differential equations though the Kutta method is Runge ..Euler method..-typical, * Various computational algorithms that attempt shortening computing time as t is assumed to be changeable or each element is changed are used, too. (→ pp. 157) Accuracy * It uses in the meaning that the expectation by the simulation is near "Accuracy of the expectation" how to be adequately expressible of the phenomenon of the reality, and this is not made an index 292 Appendix 1 Definition and explanation of term strictly in this report in the statistical though it means the difference of data. For instance, it only has to use the sum of squared deviation etc. of predictor and observed value when making it to a numeric index. (→ pp. 62) Appendix 1 Definition and explanation of term 293 Element Generically of the water quality item defined in ASM, the one that is called "State variable" in general hits this. It is substance of the simulation to calculate the time variance of the all component density after the influent quality of the all component and the initial value of each division are set. It is arranged in line writing direction in the stoichiometry matrix. (→ pp. 19) Kinetics constant Many of parameters used in ASM come under this by the constant used in the reaction rate equation. Saturation factor (In general, it is written by "Ki (I: component name)") that provides for the dependency to the maximum ratio speed (It might be called the rate constant; maximum specific growth rate μ and ratio autolysis speed b, etc.) that provides for the maxima at the speed and the element densities is typical. When the simulation is executed, it is treated as a constant, and information (As for this, the effect is described clearly in the technical report in ASM though the default value is given to each constant no part of ASM it) that the user should set. (→ pp. 20) ..doing.. ≫ ≪ Store Phenomenon that converts compound that living being took into intracellular into reserve substance (PHA, glycogen, and polyphosphate, etc.) and accumulates temporarily. In the environment to which the substrate supply and the environmental condition are steady, the living being that has the storage capacity might have a predominance in the advantage in which the metabolism like this in the condition that a no bur and these change greatly too much. The organism can be temporarily stored because the means to oxidize this in the reluctance tank where the biological phosphorus removal process , for example, a conceited reluctance-method is typical, and the organic substrate is supplied and to generate energy is limited, energy be generated by breath using this in a continuing conceited tank, and the living being that does the metabolic activity of proliferation etc. use the organic substrate preferentially. In this case, it is PAO to be necessary also for the store with the reluctance tank energy for the incorporation of the substrate and conversion into the reserve substance, and to obtain this because an inorganic energy storage material polyphosphate is hydrolyzed. In ASM2d, the store process of XPHA and XPP is defined only about XPAO to express the function of PAO. It is clarified that a lot of heterotrophic bacteriums excluding this also store the organic substrate, and modeled the proliferation also of heterotrophs XH other than PAO through the store in ASM3. (→ pp. 46) 294 Subsidence model Appendix 1 Definition and explanation of term Generic name of the model by whom phenomenon of floating material's subsiding in primary sedimentation tank and final sedimentation tank, etc. is expressed. Various dirt subsidence models to express the solid-liquid separation in the final sedimentation tank are typical. (→ pp. 67) Appendix 1 Definition and explanation of term 295 Stationary solution Value of element when value of all component (state variable) is steady (Do not change timewise) by the process of the simulation calculation. In a regular calculation into which the input condition doesn't change timewise, the state when the value of all elements settles to a constant (It is possible to consider it) value is considered to be regular. Moreover, the state that the same time variance pattern during a day came to be repeated might be considered to be regular in the case given repeating the time varying during a day in the non-stationary calculation. The influence with big SRT is given in the operating condition though the calculation period to obtaining the stationary solution depends on initial condition. (→ pp. 151) Regular calculation Simulation calculation in condition that logical input value of simulation like influent quantity, water quality, and operating condition, etc. doesn't change timewise. It might be called, "Regular simulation" and "Static simulation", etc.In the simulation using ASM, the corresponding of the condition etc. to use the day mean for all logical input values. (→ pp. 152) Technical report * The report of ASM that "Task group concerning mathematical model to support the design and driving the biological waste water treatment" of IWA made public is indicated and it uses it in this report. "Activated Sludge Models ASM1, ASM2, ASM2d and ASM3" (chapter of this task group, Scientific and Technical Report No.9, and IWA Publishing) that arranges these is published in 2000 though each version of ASM has been made public independently severally in the form of the report and the thesis. The one published in this as a technical report is indicated in this report as long as there is especially no refusal (There is a part where the correction was added, too and the content is not identical though this report is bringing of a basically original thesis etc. together). The Japanese translation book on this report is published in 2005. (→ pp. 11) Electron acceptor Generic name of compound that receives electron in redox reaction (It is reduced). The compound that discharges the electron is called "Electron donor" etc.Oxygen and the ion nitrate in breath as the terminal electron acceptor are important (It is called, "Oxygen breathing" and "Nitrate respiration", etc. respectively; The denitrification is latter one form), and in the activated sludge process, these are indicated and it uses it in this report. (→ pp. 29) 296 Tracer experiment Appendix 1 Definition and explanation of term In general, the one having aimed to examine flowing and problem data conversion such as a waterway in the sewage plant, a distribution tank, reactive tanks, and the final sedimentation tanks is only called a tracer experiment in this report though it is a generic name of the examination that examines the behavior of the material with a tracer (material that can be pursued). When the activated sludge model is used, it is possible to use it to obtain necessary information when the distribution situation of influent is confirmed, a reactive tank is evaluated problem data conversion, and the process model is constructed chiefly. Adsorption and the resolution in the research objective part are few, the measurement at high frequency is possible, and the requirement such as without the adverse effect on facilities and the processing function is requested, and the lithium chloride is used for the material used as a tracer comparatively well in our country. Besides, another of sodium chloride and dyestuff such as rhodamine are used. (→ pp. 106, 138) ..doing.. ≫ of ≪ Endoecism breath The bacillus etc. do not consume the extracellular organic substrate, the done breathing activity is indicated to the narrow sense, and because an intracellular organic substrate (reserve substance etc.) is oxidized, the energy (It is called the maintenance energy etc. ; It is thought the energy to maintain the density gradient in the inside and outside of the cell membrane and to reproduce various enzymes) to maintain the cell is obtained. However, the amount of breath that originates in other phenomena like the use of the organism generated with the activity and the bacteriolysis of the protozoan in addition to this etc. joins the respiratory rate observed on the condition that the organic substrate doesn't exist in liquor in the activated sludge process, and this is called endoecism breath to the wide sense. There are a concept etc. of the concept of ① autolysis-reproduction, the concept of ② endoecism breath, and ③ self-maintenance as an approach to model this, and ① is used in ASM1-2d (Refer to "Concept of autolysis-reproduction"). In ② adopted with ASM3, the consumption of living being's decrease and electron acceptor (oxygen and nitrate) corresponds directly, and correspondence with the endoecism respiratory rate experimentally obtained is clear. However, it is necessary to note it no confusion because the meaning of the rate constant (bH etc.) and fXI is greatly different from the model before ASM2d the numerical value of both. (→ pp. 34, 35) Input data * It uses it generically of the data that the user should set when the simulation using ASM is done. Various parameters (reactive tank pump composition and capacity etc. of the tank) for the process Appendix 1 Definition and explanation of term 297 model construction and various flow rate, the influent qualities, water temperatures, and the control values are included (In the wide sense, all parameter values are added to this; The setting of parameter value is user's responsibility matter). (→ pp. 96) 298 Appendix 1 Definition and explanation of term ≪ is ..doing.. ≫. Fermentation Generic name of reaction that oxidizes organism in reluctance by microorganism of heterotrophy type and generates energy. Intracellular organic compound is used as an electron acceptor, and it is converted into another organic compound. The acid fermentation and the methane fermentation in the anaerobic digester tank are typical in the sewage plant. Moreover, it has the meaning to PAO in the reluctance tank as the substrate supply process so that PAO that bears the biological phosphorus removal may use organic acid etc. generated by fermentation as an organic substrate. A great simplification is performed to the fermentation phenomenon of the reality as this doesn't accompany the proliferation of XH in the one that the substrate supply function to XPAO was modeled though the fermentation process is defined as a conversion process from SF by XH to SA in ASM2d. (→ pp. 32, 47) Parameter Generic name of various constant and coefficients used in model. In ASM, it is divided into three kinds (the kinetics constant, amount of theory coefficient, and the conversion factor). These are the illustration values, and the setting of parameter value has been entrusted to them by the user though the numerical value (default value) is presented in all parameters by the technical report. Work (narrow sense) to presume and to optimize parameter value on the basis of the processing results data and observed data is called a calibration. (→ pp. 19) Reaction equation Kinetics rate It is the one that the reaction rate was expressed by the expression, and substance of the kinetics of ASM. In general, it is described as a differential equation that shows the transformation rate of a certain element (state variable). It is composed of the kinetics constant and the element. In ASM, the impact combination bur of various elements is expressed by the mathematical expression based on the expression of Monod, and the factor that influences the speed of the process in seeing the reaction rate equation can be understood. (→ pp. 20) It uses it as a term that shows modeling and the mathematical expression of the reaction rate in this report though the study whole concerning the reaction rate is indicated to the wide sense. It has the frame of the content of the description of ASM with the stoichiometry. Substance is a procession of "Reaction rate equation" in each process. The term "Kinetic" that faithfully translates this into Japanese might be used though this term corresponds to "kinetics" of the original of the technical report. (→ pp. 14) Appendix 1 Definition and explanation of term 299 300 Appendix 1 Definition and explanation of term Water quality profile In this report, it uses it as a term that shows the change in the water quality along direction where a reactive tank flows. In * of reactive tank general, it is important to understand the change of an ammoniacal nitrogen, nitrate-nitrogen, and phosphoric acid Rin, etc. by the process (flowing process) in a reactive tank in the insufficiency, and to presume the phenomenon caused by each division only by referring to the treated effluent quality to understand processing status in a reactive tank. Even if it is data "Snap shot ..adoption.." ..do not consider [mizu] interval.. based on the obtaining water, the utility value is large though data that ..adoption.. did [mizu] from such an intention one by one on the basis of a mean of the day of the water quality density of each division and an actual flowing speed of a reactive tank (HRT of each division) is desirable. (→ pp. 109) Non-stationary calculation Simulation calculation in condition that logical input value of simulation like influent quantity, water quality, and operating condition, etc. changes timewise. It might be called, "Unsteady simulation" and "Dynamic simulation", etc.In the simulation using ASM, the corresponding of the simulation by real time that assumes a condition of giving the logical input value the time varying and a specific period etc. (→ pp. 152) Surface limiting type It is used by the method of expressing the rate equation of the reaction caused on the cell surface in ASM to express the substrate rate equation concentration dependency of the hydrolysis process. The substrate concentration doesn't influence the direct reaction speed like the Monod type but the substrate and amount of ratio of the living being have the functional with which the matrix part of the Monod type is replaced by "Ratio of substrate/living being" (XS /XH ratio in case of the hydrolysis process) on the basis of the idea with essential (Such a rate equation is called "Expression of Contois"). (→ pp. 23) Fitting Work to adjust parameter value and the input data of the model so that the simulation result may agree with the measurement data is indicated. The criteria of "Agreement" is various from the level to the case to use a numeric index of the error sum of squares etc. by which the shown in the figure both data is compared. Moreover, the method is also various from the trial and error by people to an automatic fitting using mathematical optimization. (→ pp. 147) Incidental model * It uses it generically of the model who is combined with the activated sludge model as a component of the activated sludge process model and used. In this report, it introduces the reactor Appendix 1 Definition and explanation of term 301 [mizukotowari] model, the oxygen transfer model, the sedimentation pond model, the pump flow model, the aerification style amount model, and the influent quality model, etc.It might be called, "Submodel". (→ pp. 8, 10) 302 Appendix 1 Definition and explanation of term Material kind Amount of substances such as elements and charges that become objects that take revenue and expenditure in ASM. Five kinds of material kinds of organism (COD), nitrogen (N), Rin (P), charge (mole+), and suspended solid (TSS) are defined for ASM2d. One kind of the material that is basic is allocated in all elements among models, and the content of other material kinds is defined by the conversion factor. As for the stoichiometry of ASM, it descends strictly on the basis of the material conservation law, and the gross weight of each material kind is constant in all the reaction processes. (→ pp. 18) Mass balance Quantitative revenue and expenditure to change by inflow going out and reaction, etc. of material (element on element, compound, and model etc.). When a certain area is defined, element of revenue and expenditure, (Each breakdown is included), and these whole is a mass balance. the outflow to the inlet flow from the outside of the material that becomes an object and the outside and the variations in thatWhen the revenue and expenditure relation has adjusted, it is said, "Revenue and expenditure is taken" etc.Moreover, the state that all the revenue and expenditure elements are understood from the measurement data etc. might be called "Revenue and expenditure shuts". It is widely used to confirm the validity of the measurement data and the condition setting, and to presume a difficult element to measure. ASM is a model based on the mass balance, all the revenue and expenditure elements are clear, and revenue and expenditure of all the material kinds and elements adjusts. Therefore, neither the calculation result nor the measurement data will agree fundamentally with that when data that there is unmatch in the mass balance is used as a logical input value. Therefore, it is assumed that the correspondence of the mass balance of the data used is confirmed before the work of the calibration etc. in this report. Moreover, measuring it directly by using the mass balance like the amount of breath and the amount etc. of the denitrification can presume the results value of a difficult variation of the material. (→ pp. 16, 127, 130) Process Generic name of reaction defined in ASM. 21 kinds of processes are defined in ASM2d. It is arranged in columnwise direction in the stoichiometry matrix, and the variation of the element by an individual process is shown in the each line. (→ pp. 18) Process model * Various incidental models are combined with the activated sludge model, and it uses it as a name of the model by whom the simulation as the activated sludge process is enabled. It means work to define and to put these incidental models when saying, "Construction of the process model", and it becomes work like the Appendix 1 Definition and explanation of term 303 composition, the capacity of a reactive tank and the final sedimentation tank, and the oxygen supplies in various ducts and conceited tanks, etc. to set the physical parameters of facilities chiefly. (→ pp. 8) 304 Appendix 1 Definition and explanation of term Conversion factor The coefficient to convert a different material cross-species is called generically by a kind of the parameter of ASM. It is used to describe the stoichiometry of the process, and it is written (c: material specific name and i: component name) as "ic,i ". There is "iTSS,i " to calculate TSS(XTSS ) from the floatage element in addition to a series of "iN,i " and "iP,i " that defines nitrogen and the phosphorus containing rate of each organism element. (→ pp. 20) Saturation factor It is used by the function of the Monod type by one of the kinetics constants. The sign of "K" is allotted usually. Mathematically, it is corresponding in the substrate concentration when the value of clause Monod becomes 0.5. Because the value of clause Monod is decided by the magnitude correlation of the substrate concentration and saturation factor, it is considered the constant that expresses intimacy of the living being to the substrate. For instance, the influence (If you do not model the floc structure) that the diffusion situation of the substrate to the inside of the activated sludge floc gives the reaction rate is typically reflected in the value of saturation factor. (→ pp. 21) Polyphosphate Generic name of polymer of phosphoric acid shown by H2PO3(HO3P) nPO4H2. Intracellular of the microorganism is generated from the phosphate ion, and accumulated as an energy storage material in the state of the granule though is industrially synthesized. The accumulated polyphosphate is used for the substrate intake, use from which the energy generation means of breath etc. is limited under the condition etc. , and the energy generation for the self-maintenance (It is converted into the phosphate ion by hydrolysis). The biological phosphorus removal is a method of removing Rin more than a usual process as a polyphosphate that accumulates PAO. Intracellular store material XPP of PAO corresponds in ASM2d, and it is modeled as an element besides XPAO. (→ pp. 29, 50) ≪ ..rolling.. [gyou ≫] Matrix The stoichiometry matrix that is the method of writing ASM is indicated and used. In the matrix, the process is arranged in columnwise direction, the element is arranged in line writing direction, and the variation of the element by the process is displayed in each cell. Therefore, a quantitative relation of the element conversion in each process is shown in the each line and Appendix 1 Definition and explanation of term 305 the element change according to all the processes will be shown in each row (Because it is a relative relation of the element variation in each process that is filled in to each cell, it is necessary to multiply the reaction rate to calculate the variation). The content of the model is understood and when modifying it, it is very useful because it is possible to have a look at the structure of the model. (→ pp. 11, 14, 27) Modeling Generic name of act making model of system of reality. It is called, "Model" etc.It is corresponding to the description of this as the expression for mathematical model on the basis of the characteristic of the system of the reality. (→ pp. 6) 306 Appendix 1 Definition and explanation of term ≪ and ..doing.. ≫ Volatile organic acid (VFA) seen in the influent of the sewage plant is only called organic acid in this report though it is defined in general as "Generic name of organic compound that shows acidity". The acetate is a subject in influent, and the propionate is often detected. Moreover, there is a case where the formic acid is detected by the inflow of the industrial effluent etc. , too. It is not included in the domestic wastewater immediately after the exhaust so much, and it is thought that it is generated by fermentation in the sewer. The density in influent greatly influences the behavior of the denitrification and the biological phosphorus removal because it is promptly used for a lot of heterotrophic bacteriums including PAO. The meaning of this element is in division with "Organism (SF ) that can use only XH", and is not strictly identical with organic acid though is modeled as SA in ASM2d. (→ pp. 31) Organic acid ..doing.. ≫ ≪ Influent segmentation * Amount of coefficient The work whole to measure and to presume the amount (density) in influent of the element defined in ASM is indicated. The presumption work of the organism element might be indicated because the center is presumption of the organism element and it be used (When it is emphasized to limit it to the organism element, it is said, "Segmentation of the influent organism element" etc.). The presumption act that uses a simple method in addition to the case to presume the OUR measurement etc. experimentally is included. When the model is used because the organism element used in ASM doesn't correspond a usual water quality parameter and directly, it is necessary to do some influent segmentations, and to define the influent quality for the simulation. The reactive tank influent quality is used as an influent quality, except when the sedimentation pond model is used the beginning. (→ pp. 113, 309, 323) theory Generic name of parameter used to describe stoichiometry in ASM. It is called, "Stoichiometric coefficient". The one to show the weight ratio rate of the element consumed by the process of the reaction and the generated element is a subject, and growth yield (YH ,YPAO ,YAUT ) in which the living being formation per amount of the substrate is defined is typical. Besides, there is coefficient (fSI ,fXI ) in which the yield of coefficient (YPO4 ,YPHA ) and an inert organism that discharges by PAO by Rin and takes and defines the relation of amount of theory of [toki] is defined. The variation of each element is shown in each cell of the stoichiometry Appendix 1 Definition and explanation of term 307 matrix by using these amount of theory coefficient and conversion factor. (→ pp. 20) 308 Phosphorus accumulation being Appendix 1 Definition and explanation of term living Loess pyrogram It is called "PAO(Phosphate Accumulating Organisms)" by the living being that becomes the subject of the biological phosphorus removal. It has the ability to take the organic substrate such as organic acids promptly by using energy that hydrolysis of the polyphosphate stored to intracellular and obtains it (The glycogen is consumed as a reducing power) under the anoxic and to store , saying that PHA (The phosphate ion is discharged into the extracellular by this process). Under aerobic condition moreover, the polyphosphate and the glycogen ..resynthesis.. proliferate by breath using this PHA (The phosphate ion in liquor is taken by this process, and Rin will be removed only as for the amount of proliferating PAO). Therefore, the state that PAO proliferates preferentially than other heterotrophs can be formed by repeating the anoxic and aerobic condition. PAO is distinguished from these in the point to show the above-mentioned behavior under aerobic condition of reluctance though a lot of living beings that accumulate the polyphosphate in intracellular as an energy storage material are known, and "Poly-P accumulation living being (Poly-P organisms)" etc. are called as for these. Living being..element..define..accumulation..polyphosphate..accumulation.. proliferation..autolysis..behavior..model..glycogen..use..reproduction ..incorporate.Moreover, the intracellular store material is defined as element (XPHA ,XPP ) besides XPAO. It is known that a part of PAO has the denitrification ability (Poly-P accumulation and proliferate by the denitrification under the anoxic condition), and the point to have modeled this is the main improvement point from ASM2 to ASM2d. (→ pp. 29, 38) In the one that the time variance of the respiratory rate (consumption speed of the electron acceptor) was graphed, the time variance curve of OUR is typical. The area enclosed by the respiratory rate curve and the time base on the loess pyrogram is corresponding in the amount of breath in the range during that time. It is a sole method of the influent segmentation and the model and the parameter estimation to discuss the model structure and parameter value that can reproduce the loess pyrogram obtained for the respiratory rate measurement. (→ pp. 310) 309 Appendix 2Sign list and translation with the original Appendix2Sign list and translation with the original The list of the sign used in the sign used with ASM2d, the list, the translation with the original of the process name, and this report is published. As for a Japanese translation, it is a Japanese translation basically book on the technical report. found.It Error! Reference source not drinks, it conforms to the term, and there is the one that the partial modification was added, too. 1.Sign and process name used with ASM2d (1)Element Element Unit English mark Soluble Components (S? ) Japanese mark Dissolubility element SO2 gO2/m3 Dissolved oxygen Dissolved oxygen SF gCOD/m3 Fermentable, readily biodegradableorganic sabstrates Fermentative organism SA gCOD/m3 Fermentation products Fermentation product (acetate etc.) SNH4 gN/m3 Ammonium plus ammonia nitrogen Ammoniacal nitrogen SNO3 gN/m3 Nitrate plus nitrite nitrogen Nitrate-nitrogen nitrogen SPO4 gP/m3 Inorganic soluble phosphorus Dissolubility inorganic phosphate SI gCOD/m3 Inert soluble organic matterial Dissolubility inert organism SALK moleHCO3-/m3 Alkalinity Alkalinity SN2 gN/m3 Dinitrogen, N2 Chokedamp easy-degradable and nitrite 310 Element Appendix2 Sign list and translation with the original Unit English mark Particulate Components (X? ) Japanese mark Floating element XI gCOD/m3 Inert particulate organic material Floating inert organism XS gCOD/m3 Slowly biodegradable substrate Slow, resolved organism XH gCOD/m3 Heterotrophic organisms Heterotroph XPAO gCOD/m3 Phosphorus-accumulating organisms: PAO Phosphorus accumulation living being XPP gCOD/m3 Poly-phosphate Polyphosphate XPHA gCOD/m3 A cell internal storage product of PAO Phosphorus accumulation living being's intracellular store material (PHA etc.) XAUT gCOD/m3 Nitrifying organisms Nitrifying bacteria XMeOH gFe(OH)3/m3 Metal-hydroxides Metal hydroxide (soluble metals) XMeP gFePO4/m3 Metal-phosphate Metallic phosphate compound XTSS gTSS/m3 Total suspended solids, TSS Suspended solid 311 Appendix 2Sign list and translation with the original (2)Parameter Parameter Unit English mark Conversion Factors Japanese mark Conversion factor IN,SI gN/gCOD N content of intert soluble COD SI Nitrogen content in SI IN,SF gN/gCOD N content of soluble substrate SF Nitrogen content in SF IN,XI gN/gCOD N content of intert particulate COD XI Nitrogen content in XI IN,XS gN/gCOD N content of particulate substrate XS Nitrogen content in XS IN,BM gN/gCOD N content of biomass XH , XPAO , XAUT Nitrogen content of living being (XH , XPAO , XAUT ) IP,SI gP/gCOD P content of intert soluble COD SI Phosphorus containing rate in SI IP,SF gP/gCOD P content of soluble substrate SF Phosphorus containing rate in SF IN,XI gP/gCOD P content of intert particulate COD XI Rin materials content in XI IN,XS gP/gCOD P content of particulate substrate XS Phosphorus containing rate in XS IN,BM gP/gCOD P content of biomass XH , XPAO , XAUT Phosphorus containing rate of living being (XH , XPAO , XAUT ) ITSS,XI gTSS/gCOD TSS to XI ratio Equivalent of TSS in XI ITSS,XS gTSS/gCOD TSS to XS ratio Equivalent of TSS in XS ITSS,BM gTSS/gCOD TSS to biomass ratio for XH , XPAO , XAUT Equivalent of TSS of living being (XH , XPAO , XAUT ) Stoichiometric Coefficients Stoichiometric coefficient fSI gCOD/gCOD Fraction of inert COD in particulate substrate Ratio from which XS is converted into SI by hydrolysis YH gCOD/gCOD Yield coefficient Growth yield of XH fXI gCOD/gCOD Fraction of inert COD generated in biomass lysis Ratio from which XH is converted into XI by autolysis YPAO gCOD/gCOD Yield coefficient (biomass/PHA) Growth XPHA YPO4 gP/gCOD Yield requirement (SPO4 release) for PHA storage SPO4 burst size necessary for store of XPHA yield of XPAO)(per 312 Parameter Appendix2 Sign list and translation with the original Unit English mark Japanese mark YPHA gCOD/gP PHA requirement for PP storage XPHA necessary for store of XPP YAUT gCOD/gN Yield coefficient (biomass/nitrate) Growth SNO3 yield of XAUT)(per Kinetics constant (hydrolysis relation) Kinetic Parameters (Hydrolysis) The maximum ratio hydrolysis rate Kh 1/d Hydrolysis rate constant ηNO3 (ηNO3,S ) - Anoxic hydrolysis reduction factor ηfe - Anaerobic factor KO2 (KO2,S ) gO2/m3 Saturation/inhibition coefficient for oxygen Saturation and constant to SO2 inhibition KNO3 (KNO3,S ) gN/m3 Saturation/inhibition coefficient for nitrate Saturation and constant to SNO3 inhibition KX gXS/gXH Saturation coefficient for particulate COD Saturation factor in hydrolysis to XS /XH hydrolysis reduction The hydrolysis rate decrease coefficient under anoxic condition The hydrolysis rate decrease coefficient under anoxic Kinetics constant (XH relation) Kinetic Parameters (XH ) μH gXS/(gXH・d) Maximum growth rate of substrate The maximum specific growth rate of XH qfe gSF/(gXH・d) Maximum rate for fermentation The maximum ratio fermentation speed ηNO3 (ηNO3,H ) - Reduction factor for denitrification The proliferation rate decrease coefficient of XH under anoxic condition bH 1/d Rate constant for lysis and decay Ratio autolysis speed of XH KO2 (KO2,H ) gO2/m3 Saturation/inhibition coefficient for oxygen Saturation and constant to SO2 KF gCOD/m3 Saturation coefficient for growth on SF Saturation factor in proliferation of XH to SF Kfe gCOD/m3 Saturation conefficient fermentatin of SF Saturation factor in fermentation to SF KA (KA,H ) gCOD/m3 Saturation coefficient for growth on SA Saturation factor to SA KNO3 (KNO3,H ) gN/m3 Saturation/inhibition coefficient for nitrate Saturation and constant to SNO3 KNH4 (KNH4,H ) gN/m3 Saturation coefficient for ammonium (nutrient) Saturation factor to SNH4 for inhibition inhibition 313 Appendix 2Sign list and translation with the original Parameter Unit English mark Japanese mark KP (KP,H ) gP/m3 Saturation coefficient for phosphate (nutrient) Saturation factor to SPO4 KALK (KALK,H ) moleHCO3-/m3 Saturation coefficient for alkalinity Saturation factor to SALK Kinetics constant (XPAO relation) Kinetic Parameters (XPAO ) qPHA gXPHA/ (gXPAO・d) Rate constant for storage of XPHA The maximum ratio store speed of XPAO qPP gXPP/(gXPAO ・d) Rate constant for storage of XPP The maximum ratio store speed of XPP μPAO 1/d Maximum growth rate of PAO The maximum specific growth rate of XPAO ηNO3 (ηNO3,P ) - Reduction factor for anoxic activity Proliferation of XPAO under anoxic condition and store rate decrease coefficient of XPP bPAO 1/d Rate for lysis of XPAO Ratio autolysis speed of XPAO bPP 1/d Rate for lysis of XPP Ratio autolysis speed of XPP bPHA 1/d Rate for lysis of XPHA Ratio autolysis speed of XPHA KO2 (KO2,P ) gO2/m3 Saturation/inhibition coefficient for oxygen Saturation and constant to SO2 inhibition KNO3 (KNO3,P ) gN/m3 Saturation/inhibition coefficient for nitrate Saturation and constant to SNO3 inhibition KA (KA,P ) gCOD/m3 Saturation coefficient for SA Saturation factor to SA KNH4 (KNH4,P ) gN/m3 Saturation coefficient for ammonium (nutrient) Saturation factor to SNH4 KPS gP/m3 Saturation coefficient phosphorous in storage of PP for Saturation factor in store of XPP to SPO4 KP gP/m3 Saturation coefficient for phosphate (nutrient) Saturation factor in proliferation of XPAO to SPO4 KALK (KALK,P ) moleHCO3-/m3 Saturation coefficient for alkalinity Saturation factor to SALK KPP gXPP/(gXPAO ・d) Saturation coefficient poly-phosphate KMAX gXPP/(gXPAO ・d) Maximum ratio of XPP /XPAO Maximum XPP content of XPHA KIPP gXPP/(gXPAO ・d) Inhibition coefficient for PP storage Inhibition constant in store of XPP KPHA gXPHA / (gXPAO・d) Saturation coefficient for PHA XPHA/ XPAO for XPP in store of XPHA/ Saturation factor to XPAO Saturation factor to 314 Appendix2 Sign list and translation with the original Parameter Unit English mark Kinetic Parameters (XAUT ) Kinetics constant (XAUT relation) μAUT 1/d Maximum growth rate of XAUT The maximum specific growth rate of XAUT bAUT 1/d Decay rate of XAUT Ratio autolysis speed of XAUT KO2 (KO2,A ) gO2/m3 Saturation coefficient for oxygen Saturation factor to SO2 KNH4 (KNH4,A ) gN/m3 Saturation coefficient for ammonium (substrate) Saturation factor to SNH4 KALK (KALK,A ) moleHCO3-/m3 Saturation coefficient for alkalinity Saturation factor to SALK KP (KP,A ) gP/m3 Saturation coefficient for phosphate (nutrient) Saturation factor to SPO4 Kinetic Parameters (precipitation) - Japanese mark Kinetics constant (cohesion relation of Rin) kPRE m 3/ (gFe(OH)3・d) Rate constant for P precipitation SPO4 insoluble constant kRED 1/d Rate constant for redissolution XMeP constant KALK (KALK,M ) moleHCO3-/m3 Saturation coefficient for alkalinity Saturation factor to SAL K making solubilization In the technical report, the sign that shows the type of the process in the suffix writing if necessary is used in this report in addition because saturation factor in each process might be actually discussed, a different numerical value be set, and it is confusing though the same sign (KO2 ,KNO3 ,KNH4 ,KP ,KALK ) is used to write saturation and the inhibition constant in the whole process (S: hydrolysis, H:XH, P:XPAO, A:XAUT, and M: Rin's coagulating sedimentation). These are shown by parenthesis writing in the above table. rate rate Appendix 2Sign list and translation with the original 315 (3)Process Process name (English mark) Process name (Japanese mark) Hydrolysis processes Hydrolysis relation 1 Aerobic hydrolysis Hydrolysis under aerobic condition 2 Anoxic hydrolysis Hydrolysis under anoxic condition 3 Anaerobic hydrolysis Hydrolysis under anoxic) XH relation Heterotrophic organisms: XH 4 Growth on fermentable substrates SF Proliferation under aerobic condition of XH of which substrate is SF 5 Growth on fermentaion products, SA Proliferation under aerobic condition of XH of which substrate is SA Denitrification with fermentable Proliferation under anoxic condition of XH of which substrate is SF (denitrification) Denitrification with fermentaion Proliferation under anoxic condition of XH of which substrate is SA (denitrification) 6 substrates, S F 7 products, S A 8 Fermentation Fermentation 9 Lysis Autolysis of XH Phosphorous-accumulatingorganisms: XPAO XPAO relation 10 Storage of XPHA Store of XPHA 11 Aerobic storage of XPP Store of XPP under aerobic condition 12 Anoxic storage of XPP Store of XPP under anoxic condition 13 Aerobic growth on XPHA Proliferation of XPAO under aerobic condition 14 Anoxic growth on XPHA Proliferation of XPAO under anoxic condition 15 Lysis of XPAO Autolysis of XPAO 16 Lysis of XPP Autolysis of XPP 17 Lysis of XPHA Autolysis of XPHA 316 Appendix2 Sign list and translation with the original Process name (English mark) Process name (Japanese mark) Nitrifying organisms: XAUT XAUT relation 18 Aerobic growth of XAUT Proliferation of XAUT 19 Lysis of XAUT Autolysis of XAUT Simultaneous precipitation of phosphorus with ferric hydroxide Rin's chemical precipitation 20 Precipitation Making of SPO4 insoluble 21 Redissolution Solubilization of XMeP Appendix 2Sign list and translation with the original 317 2.Other sign and abbreviation, etc. (1)Sign Sign Unit Content ak - Step inflow ratio to k steps bH' 1/d Ratio autolysis speed of XH that corrects regeneration of XH CCOD,degrad gCOD/m3 Amount of CODCr that XH uses (influent concentration conversion) CCOD,in gCOD/m3 CODCr density of influent CCOD,out gCOD/m3 CODCr density of treated water CN,nit gN/m3 Nitrogen concentration of influent for nitrification CNO3,in gN/m3 Density of influent (nitrate-nitrogen + nitrite nitrogen) CNO3,out gN/m3 Density of treated water (nitrate-nitrogen + nitrite nitrogen) COrgN,in gN/m3 Organic nitrogen concentration of influent COrgP,in gP/m3 Organic phosphorus concentration of influent CTKN,in gN/m3 Kjeldahl character nitrogen concentration of influent CTKN,out gN/m3 Kjeldahl character nitrogen concentration of treated water CTN,in gN/m3 Density of total nitrogen of influent CTN,out gN/m3 Density of total nitrogen of treated water CTP,in gP/m3 Density of total phosphorus of influent CTP,out gP/m3 Density of total phosphorus of treated water CODdenit gCOD/d Amount of denitrification breath CODO2resp gCOD/d Amount of oxygen breathing fCOD,X gCOD/gSS CODCr content of dirt 318 Appendix2 Sign list and translation with the original Sign Unit Content fN,X gN/gSS Nitrogen content of dirt fP,X gP/gSS Phosphorus containing rate of dirt H m Depth of reactive tank L m The total extension of reactive tank (direction of flowing) N Tank Tank number of partitions Ndenit gN/d Amount of denitrification Nnit gN/d Amount of nitrification Q m3/d Flow rate and quantity of water to be treated QW m3/d Excess sludge flow rate r - Dirt sending back ratio)(per influent quantity R - Internal circulation ratio)(per influent quantity dSO2,aer /dt gO2 /d Oxygen supply speed SO2,s gO2 /m3 Saturation dissolved oxygen concentration V m3 Capacity of reactive tank W m Width of reactive tank X gSS/m3, gTSS/m3 Solid concentration XSS,ave gSS/m3 Average SS density of reactive tank XSS,in gSS/m3 SS density of influent XSS,k gSS/m3 SS density of k in reactive tank steps XSS,r gSS/m3 SS density of sending back dirt XSS,T1 gSS/m3 SS density of the first division in reactive tank Appendix 2Sign list and translation with the original Sign Unit Content XSS,W gSS/m3 SS density of excess sludge ΘX d SRT τ d HRT 319 320 Appendix2 Sign list and translation with the original (2)Abbreviation Abbreviation English mark Japanese mark ASRT Aerobic solids retention time Aerobic solid dwell time ASM Activated Sludge Model Activated sludge model BODu Ultimate BOD Ultimate BOD CFD Computational fluid dynamics Computational fluid dynamics GAO Glycogen accumulating organisms Glycogen being HRT Hydraulic retention time Hydraulic residence time IWA International Water Association International water society PAO Phosphorous accumulating organisms Phosphorus accumulation living being PHA Polyhydroxy alkanoate Polyhydroxyalkanoate polyhydroxy fatty acid PHB Poly-Β-hydroxy butyrate Poly-Β hydroxybutyrate SRT Solids retention time Solid dwell time VFA Volatile fatty acid Volatile organic acid accumulation Bibliography 2A) "Activated sludge model ASM1, ASM2, ASM2d, and ASM3" [**] Mino ([**yaku]): Environmental newspaper and 2005. living and Appendix 3Experimental method for influent organic matter fractuation 321 Appendix3Experimental fractionation of influent organism element 1.Outline In ASM2d, the organism is classified into index (SA ,SF ,SI ,XS ,XI ,XH ) based on peculiar biodegradability and it handles it. Therefore, when the influent quality for the simulation is set, the density of each organism element will be set. The composition ratio of these elements is ("because it differs greatly according to the waste treatment facility. Error! Reference source not found. Error! Reference source not found.Refer to") It is important to do the setting that reflects the realities of the object facilities (It is based on = measurement data). The method of presuming the organism element density in influent can be divided roughly into the method of (a) experimental presumption and the method of converting and converting (b) from other water quality parameters. Here, it introduces the one executed as an example of the method mainly composed of the measurement of typical oxygen utilization speed (OUR) in JS as an experimental estimation method. When the activated sludge model is used in the business, it is necessarily profitable to understand the outline because the necessity for adopting such a complex method is an estimation method that comparatively agrees with the definition of the organism element of a no bur and the model inside according to the purpose. Moreover, it is general for the targeted influent to characterize influent when general is different from the municipal sewage (The industrial effluent of the amount that cannot be disregarded mixes) by the method of evaluating such "Biodegradability", and to add the correction to the structure of the model if necessary. 2.Principle of presumption using OUR OUR measures the oxygen utilization speed of the supply water sample as its name suggests. Two NH4-N as the substrate of the organism of (a) possible biodegradation and the (b) nitrification is a subject for general influent of the sewage plant assuming that with a biological oxygen consumption of the elements. Therefore, if OUR is measured on the condition of the addition of allylthio urea (ATU) etc. and controlling nitrification, OUR according to the consumption of the organism of a possible 322 Appendix 3Experimental method for influent organic matter fractuation biodegradation is chiefly appreciable. Here, because OUR reflects the consumption speed of the organism by the living being, the difference of the degradation rate of the organism can be evaluated by the difference of the OUR level. Because the degradation rate of the organism is decided depending on a biomass in which it is borne and the kinetics characteristic, these can be presumed from measurements of OUR. Moreover, because the time integration value of OUR in a specific section corresponds to the quantity of oxygen consumed there when OUR of the sample to which the consumption of the organism progresses is successively measured, presuming the amount of the organism consumed in the section becomes possible. Appendix 3Experimental method for influent organic matter fractuation 323 The one that the time variance curve of OUR when the dirt that exists in the endoecism respiratory condition is added to the drainage sample including organisms of possible biodegradations of second classes named SS and XS as a typical example, and OUR is measured successively (This is called "Loess pyrogram" etc.) was shown typicalFigure A3. 1..going out.. .Then, "Interpretation" is as follows on the basis of the description of ASM2d. this ・ OUR increases rapidly after adding influent. This reflects the oxygen consumption according to the proliferation of XH using SS in influent. ・ This high OUR continues until SS becomes a low concentration and this becomes the rate limiting factor of the proliferation process. OUR has increased gradually for this period because XH increases by proliferation. ・ OUR decreases rapidly because the supply of SS by hydrolysis of XS becomes the limiting of the proliferation of XH when SS becomes a low concentration. ・ It arrives at a first endoecism breath level when OUR decreases as XS is consumed, and this dries up. Among these, (of the oxygen consumption in the section where first OUR is high. Figure A3. 1..It corresponds to ..drinking.. area of [ko] ..saying.. [mou] multiplication part).. ..oxygen consumption according to consumption of SS of the influent origin.. (of the oxygen consumption in the hydrolysis limiting section afterwards. Figure A3. 1Drink..say..multiply..area..correspond..influent..origin..cause..consumption..oxygen consumption..consider..with..each..section..consume..organism..amount..that is..influent..amount..calculation..for instance.Figure A3. 1If the oxygen consumption according to the consumption of SS of the influent or more origin is calculated, the amount of SS in influent can be presumed from following equation. S S ,in 1 OURS S dt 1 YH Expression (A3. 1) SS and in ..here..: Growth yield - of OUR mgO2 /L/h according to the consumption of SS density mgCOD/L of the influent origin and OURSs :SS and in and YH:XH and ..going out.. .. In this presumption, YH is necessary as already-known information. Because appearance YH rises according to the contribution when the store process of SS 324 Appendix 3Experimental method for influent organic matter fractuation described with ASM3 progresses at the same time, this can be corrected though it is natural to use the default value of ASM2d. Appendix 3Experimental method for influent organic matter fractuation ①下水添加後の高いOURの挙動 基質制限が無いXHの増殖を反映 → μH、XHの推定 ⑤高OUR区間の面積 325 ②OUR急減時の挙動 SS枯渇による増殖速度低下を反映 → KSの推定 ⑥底部OURの面積 下水中のXS由来のSS を利用した 増殖に伴う酸素消費量に相当 → 下水中のXSの推定 下水中のSSを利用した増殖に伴う 酸素消費量に相当 → 下水中のSSの推定 → YHの推定(SS既知の場合) ③SS 枯渇後のOURの挙動 加水分解速度の変化を反映 → Kh、KX、XSの推定 ④SS およびXS枯渇後のOURの挙動 OUR XHの自己分解を反映 → bHの推定 OURの時間変化曲線 下水添加 時間 SSを利用した増殖 XS の加水分解 XHの自己分解 OURを律している反応プロセス Figure A3. 1Conceptual diagram of the interpretation method based on OUR measurement result that uses influent and dirt and ASM2d On the other hand, high OUR(OUR0 ) immediately after addition of influent is ..(.. expression in which the organic substrate reflects proliferation rate (μH ・XH ) of XH in the condition of not becoming a limiting. OUR0 1 YH H X H YH Expression (A3. 2))。 Expression (A3. 2) Therefore, if YH and μH are assumed (default value etc.), the expression. Expression (A3. 2)The amount of XH in the sample or more can be presumed. It 326 Appendix 3Experimental method for influent organic matter fractuation is also possible to presume the amount of XH in influent if a similar measurement is done by not adding dirt, and using only the living being in influent. 327 Appendix 3Experimental method for influent organic matter fractuation XH OUR0 YH 1 YH H Expression (A3. 3) Therefore, SS, XS, and the XH density of influent can fundamentally be presumed by using the OUR measurement. However, because the dirt when a long measuring time (for instance, several days) begins to be needed, and to measure it should be strictly assumed to be "Endoecism respiratory condition" to presume XS by the above-mentioned method, application is actually difficult. Therefore, presumption like the above-mentioned limits to SS, and presumes the density where the model can reproduce the loess pyrogram with the fitting about XS. Because any inactive ingredients (SI ,XI ) not biologically used at all do not influence the measurement result of OUR as a definition, it is fundamentally impossible to presume these. OUR single measurement it As for "Interpretation" of the OUR measurement result, like the above-mentioned, it is necessary to note a point different depending on the assumed model. For instance, in the model including the store process like ASM3Figure A3. 1Because proliferation progresses at the same time there, too presuming the oxygen consumption that originates in an individual process becomes very difficult though it is interpreted that high initial OUR in [noyouna] [re] spiro gram strongly reflected the store process from proliferation. It is in fact impossible that use and hydrolysis of the reserve substance individually similarly handle these of the behavior after SS dries up because simulataneous progress. Moreover, the expression. Expression (A3. 3)Then, there is no paragraph according to autolysis because it assumes "Concept of autolysis-reproduction" adopted with ASM2d as a description of living being's autolysis. When "Concept of endoecism breath" like ASM3 is assumed, it is necessary to add the paragraph where OUR according to autolysis is expressed. On the other hand, when the living being that has the storage capacity in the dirt of the measuring object excels, and OUR of measuring initial depends on the store process strongly actually, a part of SS used will be considered to be XS in the above-mentioned method based on ASM2d that doesn't consider the store by XH. Error! Reference source not found.。 It is undesirable to undervalue the amount of the organism promptly used from the aspect that describes the amount of the organism actually used by XH in a specific section (It is included to express the competition over the organic substrate between XH and XPAO) though the use of the store organism by XH becomes such presumption from the standpoint of dividing SS and XS according to the difference of the respiratory rate 328 Appendix 3Experimental method for influent organic matter fractuation because it is included in the hydrolysis process. It is necessary to temper with the oxygen consumption when the store organism in the section after OUR decreases is used to proliferate to understand the amount of the organism truly used here, and it is in fact impossible to distinguish this from the oxygen consumption according to the use of the organism of the hydrolysis origin on the loess pyrogram when XS coexists. The measurement that adds the acetate of the known amount is concurrently executed, and the process of presuming yield used to calculate SS from the difference of an initial oxygen consumption by the presence of the acetate addition is built in from such circumstances in the following estimation methods. In this, corresponding is "to the correction of the contribution of the store of measuring initial in the high OUR section. Error! Reference source not found. Error! Reference source not found.In investigation of actual conditions intended for 16 places in the sewage plant shown in", estimate value in yield is a value whose YH of 0.69-0.84(0.77 on the average) and ASM2d is obviously higher than that of default value (0.625). Actually, simulataneous progress to proliferation that uses an external substrate directly and proliferation, etc. that use the store and the store organism in the high OUR section of (b) initial with a possibility different from time when the growth yield when the (a) acetate is used uses other organisms in drainage. Because it doesn't have the rationality that the proper move method is enough as there is no guarantee that can do the extrapolation of yield when the acetate is added because each contribution is different depending on the amount of the organism of experimenting initial, it proposes to consider the estimated result of SF to be the uncertain one, and to add to the adjustment candidate at the calibration in report. 329 Appendix 3Experimental method for influent organic matter fractuation 3.Fractionation procedure (1)Estimation method of each element The estimation method of each organism elementTable A3. 1It is as [ni] is brought together. Table A3. 1Estimation method of influent organism element Element Necessary sample Estimation method SA (fermentation product) Measurements propionate). SF (easily decomposable organic matter that can be fermented) After SS is presumed from OUR (influent + dirt) measurement result, SA is subtracted. SI (dissolubility inert organism) XI (floating inert organism) XS (slow, resolved organism) Fitting to OUR (influent + dirt) measurement result. Or, it presumes from ultimate BOD. Influent sending back dirt XH (heterotroph) Presumption from measurement result. Influent Other living being (XPAO , XPHA , XAUT ) elements of organic acid (acetate + Dissolubility CODCr measurements of treated water. (Dissolubility BOD5 and NO2-N are corrected if necessary. ) Another element is subtracted from total CODCr of influent. OUR (influent single) Influent Influent sending back dirt Treated water Influent Disregard (In the simulation, the low concentration is set). (2)Collection of sample The following samples are collected. All, it is basic to use the one of facilities for the simulation. Because the density may change into elements such as SA and SF even if it is the refrigeration condition of 4℃ under when time has passed since it collectedError! Reference source not found., Error! Reference source not found.、The following measuring water qualities and the OUR measurement operations are promptly executed. ・ Influent ・ Sending back dirt ・ Treated water 330 Appendix 3Experimental method for influent organic matter fractuation (3)Measuring water quality A at least necessary water analyses are as follows for the organism element segmentation. ・ Influent: CODCr and organic acid (acetate and propionate) ・ Treated water: Dissolubility CODCr, dissolubility BOD5, and NO2-N (4)OUR measurement Device (..device measured successively DO density of attendant test solution to monitor OUR necessary.. Appendix 3Experimental method for influent organic matter fractuation 331 Table A3. 2)。For the unit that measures the DO density, the near sealing up structural one is used to avoid the dissolution of oxygen from the atmosphere. Measurement condition 332 Appendix 3Experimental method for influent organic matter fractuation Table A3. 3The measurement on four [ni] conditions of showing (condition 1-4) is assumed to be basic. However, condition 2 is omitted when it is inside to be able to disregard the amount the organism dirt (An empty aerification enough for prior is done) and when YH is not corrected, condition 3 can be omitted. Additionally, the one for which each condition is used as a common measurement condition in JS is as follows. ・ Mixing ratio of influent and sending back dirt: 10:1 (capacitor ratio) ・ Water temperature: 20±1℃ ・ Range of measurement DO: 3-6 Mg/L ・ Uptake of DO data: 0.5s pitch (Averaging what of each 10s is output). Appendix 3Experimental method for influent organic matter fractuation 333 Table A3. 2Example of device for OUR measurement3C) Example of device Feature ・Culture and the measurement are done in the airtight container that inserts the DO electrode. The aerification under the measurement accomplishes the reaction by not doing, and using only first stage DO ([Mizu] and ..supply.. ..trial.. diluent are aerated beforehand). (a) One tank type (There is no DO supply). パソコン DO計 - The device is easy (The respiratory rate measurement unit of DO meter on the market can be used as it is). - The DO monitor is not interrupted. To make all react in a narrow DO area of ×(Even if it is the maximum, it is saturation DO level), the absolute value of OUR becomes small. The performance of the DO electrode and the influence of the stir are received easily, and data varies easily. Because it is difficult to expect best dilution ratio for × prior, the preliminary measurement is needed. The influence with × dilution water might be received. The collection of the sample for the measuring water quality is impossible while × is measured. DO電極 スターラ (b) One tank type (DO supply and exist). エアポンプ DO計 DO電極 スターラ 制御 ユニット パソコン ・ (a)The one that [ni] aeration function was added. ON/OFF of the aerification is repeated so that DO may enter the setting range. It is convenient to turn the aerification on and off by the automatic operation by the output from the DO meter. - It is not necessary to dilute the sample. - It is possible to measure it for a long time. The escape of the air at × is needed, and it is difficult to make it to a complete encapsulated type. The data under aerating becomes invalid as for ×. The device is needed to collect the sample for the measuring water quality while measuring it in × the device. 334 Appendix 3Experimental method for influent organic matter fractuation (c) Two tank type エアポンプ DO計 パソコン DO電極 送液ポンプ スターラ ・ The chamber that measures OUR is independently set up, and culture and the DO measurement are done in another tank. The supply water sample in the chamber is replaced at regular intervals, and the DO monitor for set time is done (Or, when DO becomes below the definite value, the supply tries and replaces [mizu]). In a respiratory rate meter on the market, there are a lot of one of this type. - It is not necessary to dilute the sample. - The measurement of a long term is possible. - Because DO is measured in the chamber of the small capacity, the influence of the stir condition is not received easily. - The sample for the measuring water quality can be easily collected. - It is possible to correspond also to the substrate addition continuous running and measuring it. × device becomes complex. Appendix 3Experimental method for influent organic matter fractuation 335 Table A3. 3Condition of OUR measurement for influent organism element segmentation Conditio n Influent 1 - 2 Ion Exchang e water - 3 - 4 - Sending back Dirt One ..ac etate *.. ATU*2 Intention - - Presumption of SS and XS. - - Correction of amount organism of dirt. - - YH correction. --3 - Presumption of XH. - of - 1 It is 20 as for the sodium acetate liquor About mgCOD/L (influent concentration conversion) addition. - 2 It is 8 for the nitrification inhibition About mgATU/L is added. - 3 Addition when SS of influent is low concentration. Two influent with different SS density were targeted as an example of the measurement result of condition 1-3. 336 Appendix 3Experimental method for influent organic matter fractuation Table A3. 3[Ni] is shown. In sample ①, the section where OUR is high appears clearly after beginning to measure condition 1, and it is clear that this originates in SS of influent in condition 3 of adding the acetate because the duration is long. On the other hand, it is a result that changing OUR is not so seen in condition 1 in sample ②. The section that clearly seems that SS originating it appears in condition 3, and I understand the main stream beginning of irrigation did not truly contain SS only from this result because it becomes similar behavior when the mixing ratio of influent and dirt is too large though unclearness whether SS exists really remains. Thus, condition 3 might provide not only the purpose to correct YH but also useful information to interpret the behavior of condition 1. Calculation of OUR The decline (OUR) is calculated from the collecting DO data. Interval time of the calculation comes to receive the influence of the noise of the measurement easily though should reduce enough to changing OUR if it sets it small too much. In JS, the above-mentioned uptake data is basically used and 1 Average OUR between min is calculated. Appendix 3Experimental method for influent organic matter fractuation 337 OUR [mg/(L·h)] 30 条件3(流入水+酢酸+返送汚泥) 条件1(流入水+返送汚泥) 20 条件2(イオン交換水+汚泥) 10 0 0 1 2 3 4 Time [h] (a) Sample ①(case with high SS density of influent) OUR [mg/(L·h)] 30 条件3(流入水+酢酸+返送汚泥) 20 条件1(流入水+返送汚泥) 条件2(イオン交換水+汚泥) 10 0 0 1 2 3 4 Time [h] (b) Sample ②(case with low SS density of influent) Figure A3. 2Example of OUR measurement result (condition 1-3) Presumption of each element density Each organism element density of influent is presumed from the above-mentioned measuring water quality and the OUR measurement result. ・ SA: The organic acid measurement result of influent is considered to be SA. ・ (..the calculation of the oxygen consumption considered to be dependence on the consumption of SS from the loess pyrogram of SS:OUR measurement condition 1... Figure A3. 1Reference) Expression Expression (A3. 1)[Niyori] SS density is calculated. However, the corresponding oxygen consumption is corrected from the one of condition 1 to this by the balance Lycium chinense when judged that the 338 Appendix 3Experimental method for influent organic matter fractuation organism that was able to be used in dirt remained from the OUR measurement result of single dirt (condition 2). Moreover, when YH is corrected from the measurement result of condition 3 adding the acetate of the known amount, following equation is used. Appendix 3Experimental method for influent organic matter fractuation YH 1 OU S S ,3 OU S S ,1 CODac 339 Expression (A3. 4) OUSs and 1 ..here..: oxygen consumption mgO2 /L according to the SS consumption in condition 1 and OUSs and 3: oxygen consumption mgO2 /L according to the SS consumption in condition 3 and CODac: Dosage mgCOD/L of the acetate and ..going out.. .. ・ SA is and is calculated from SF:SS subtracting. S F ,in S S ,in S A,in Expression (A3. 5) ・ SI: Dissolubility CODCr of the treated water is considered to be SI. However, when dissolubility BOD5 and NO2-N of the amount that cannot be disregarded in the treated water remain, it corrects it by following equation. S I ,in S CODCr ,out S BOD 5 ,out 1.14 NO2 ,out Expression (A3. 6) SI and in ..here..: SI density mgCOD/L of influent and S-CODCr and out: dissolubility CODCr mgCOD/L of the treated water and S-BOD5 and out: dissolubility BOD5 mgBOD/L of the treated water and NO2 and out: NO2-N density mgN/L of the treated water and ..going out.. .. ・ XI:Another element is and is calculated from total CODCr of influent subtracting. X I ,in CODCr ,in S A,in S F ,in S I ,in X S ,in X H ,in Expression (A3. 7) ・ To the loess pyrogram of XS:OUR measurement condition 1(Or, condition 3) 340 Appendix 3Experimental method for influent organic matter fractuation Table A3. 4The shown simple organism removal model of [ni] is done in the fitting and presumed. This model omitted the (a) fermentation process to the description according to the organism removal of ASM2d, and gave autolysis of (b) XH, and limiting factors (SO2 ,SNH4 ,SPO4 ,SALK ) other than the organism element were given by "Concept of endoecism breath" in the description (Regeneration of XS was not considered) and the (c) rate equation and all simplifications of omission were given. Appendix 3Experimental method for influent organic matter fractuation 341 Table A3. 4Structure of simple model used by presuming XS Element Process Reaction rate equation SO2 Hydrolysis Proliferation of XH -(1- YH )/ YH Endoecism breath -(1- fXI ) SS XS 1 -1 XH -1/ YH Kh XS / XH XH KX XS / XH 1 H SS XH K S SS -1 b XH 区間 II 区間 I 区間 III OUR [mg/(L·h)] 30 調整② 20 調整③ 実測値 10 デフォルト 調整① 0 0 1 2 3 4 Time [h] Figure A3. 3Example of fitting for XS presumption ・ The example of the fitting processFigure A3. 3[Ni] result of showing is shown below. ① The SS density separately presumed and XS and the XH density arbitrarily set are given as initial condition, and the calculation using the default value is done to all parameters ("Default" curve). ② To reproduce the inclination of the section (section II) where OUR decreases, saturation factor KS according to SS is adjusted ("Adjustment ①" curve). ③ To reproduce the terminal in the OUR level and the same section of the section (section I) considered to be progress of the consumption of SS of the influent origin, the XH density and μH of measuring initial are adjusted 342 Appendix 3Experimental method for influent organic matter fractuation ("Adjustment ②" curve). ④ To reproduce the behavior after OUR decreases, an initial XS density is adjusted ("Adjustment ③" curve). It executes it if necessary on two or more conditions of changing kinetics constant (Kh ,KX ) according to hydrolysis. ⑤ ① -③ is repeated if necessary. ・ XH: As the XH density that can reproduce this, OUR(OUR0 ) when beginning to measure it from the result of measurement condition ④ OUR is extrapolated, and the expression. Expression (A3. 3)[Suru] of ..twining.. calculation (Figure A3. 4)。 O U R [m g/l/h] 40 30 OUR0 20 10 0 0 1 Tim e [h] 2 3 Figure A3. 4Example of OUR measurement result for XH presumption Bibliography 3A) Hiroshi Itokawa [**], Toshikazu Hashimoto, and Hitoshi Nakazawa: Fundamental discussion concerning method of influent organic constituent segmentation for activated sludge model, the 39th drainage research symposium lecture collection, and pp. 221-223,2002. 3B) Hiroshi Itokawa [**], Toshikazu Hashimoto, and Takao Murakami: Measurement of segmentation of organic constituent in drainage for activated sludge model, environmental engineering study collection, Vol.40, and pp.41-52,2003. 3C) [**] Mino: The 3rd influent segmentation, calibration, journal "EICA", Vol.8, No.1, and pp.61-68,2003. 3D) "Activated sludge model ASM1, ASM2, ASM2d, and ASM3" [**] Mino ([**yaku]): Environmental newspaper and 2005. 3E) Dircks,K., Pind,P.F., Mosbak,H. and Henze,M.: Yield determination by respirometry- The possible influence of storage under aerobic conditions in activated sludge. Water SA, Vol.25, Appendix 3Experimental method for influent organic matter fractuation 343 pp.69-74, 1999. 3F) Guisasola,A., Sin,G., Baeza,J.A., Carrera,J. and Vanrolleghem,P.A.: Limitations of ASM1 and ASM3: a comparison based on batch oxygen uptake rate profiles from different full-scale wastewater treatment plants. Wat.Sci.Tech., Vol.52, No.10-11, pp.69-77, 2005. 3G) Sperandio,M., Urbain,V., Ginestet,P., Audic,M.J. and Paul,E.: Application of COD fractionation by a new combined technique: comparison of various wastewaters and sources of variability. Wat.Sci.Tech., Vol.43, No.1, pp.181-190, 2001. Appendix 4Investigation of actual conditions of influent organic matter characterization 345 Appendix4Investigation of actual conditions of influent organism element 1.Outline It is necessary to construct the process model based on the composition and the parameter of the object facilities when the process simulation using ASM2d is done, and to set the influent quantity, the water quality, the operating condition, and the water temperature, etc. as input data. Especially, a peculiar index to ASM2d of which the unit is CODCr is used for the organism element, and the water quality data that we are using in the place of a usual business cannot be used directly though it is necessary to give the density of each element defined by ASM2d about the influent quality. The typical one is "as the method for the grasp of the organism element density in influent. Error! Reference source not found. Error! Reference source not found.A complex experiment operation and the analysis of the result are needed compared with a usual measuring water quality, and it is unsuitable for a daily monitor though it is the one using the measurement of oxygen utilization speed (OUR) introduces by". Then, the discussion about a simple estimation method using the measurement data that can effectively use the water quality data daily collected in the sewage plant in our country, and build it in usual water analyses situation of the waste treatment facility easily becomes important. A lot of cases where the segmentation is actually operated on the assumption of use with ASM2d are necessary for that for the influent of the sewage plant in our country. Various investigation of actual conditions is executed from such a background from 2001 to 15 years in JS, and it introduces a part of the result here. ・ A relativity investigation of CODCr and other carbon-estimating parameters: Other carbon-estimating parameters (BOD5,CODMn) along with CODCr were measured for influent in 90 places in the sewage plant, and these correlations were investigated. ・ The investigation of actual conditions of the organism element composition: It investigated whether the segmentation of the organism element of 18 kinds of influent samples that collected in 16 places in the sewage plant mainly composed of the OUR measurement was operated, and how the organism element 346 Appendix 4Investigation of actual conditions of influent organic matter characterization composition of influent was the different between the treatments. ・ The time varying situation investigation of the organism element composition: The change situation of the influent organism element composition of three kinds of Thymus vulgaris span (time varying, change on a day of the week, and secular fluctuation) was investigated for four places in the sewage plant. Appendix 4Investigation of actual conditions of influent organic matter characterization 347 2.Relativity investigation of CODCr and other carbon-estimating parameters (1)Purpose CODCr is the only carbon-estimating parameter used with ASM2d. On the other hand, you may say that it is none at all that this index is used in the place of the business according to the design and driving the sewage plant because this index is not adopted as an item of the water quality regulation according to the organism in our country. If you can presume CODCr by using these because BOD5 and CODMn of influent are regularly measured on the other hand in all sewage plantsError! Reference source not found.、One load when the activated sludge model is used will be reduced. Moreover, the method of difficult the collection of the data of the influent quality at the stage of the facilities design actually, and appropriately presuming CODCr from the design water quality of BOD5 and CODMn may be called an indispensable matter to use the activated sludge model then. Investigation of actual conditions aiming to find the method of presuming CODCr from the carbon-estimating parameter such as BOD5 and CODMn usually used from such a viewpoint intended for the influent of a lot of sewage plants. (2)Search procedure Object facilities 90 places of the Japanese whole country in the sewage plant were targeted. So as not to cause the deviation in the geographical distribution, the exclusion scheme, and the facilities scale, etc. , the object facilities were selected. The distribution of the facilities scaleFigure A4. 1It is as showing [ni]. Moreover, facilities of the separate system occupy 71 places and many, and, additionally, the confluence type and the complete confluence type where the separate system including the confluence district contains the branch district by 11 places are 4 places in the exclusion scheme respectively. Method of sampling [Mizu] of the multiple (2-17 times) ..adoption.. was done in each facilities, and various carbon-estimating parameters were measured. As a rule, the collection part of the sample was made reactive tank influent. The method of collecting the sample is a spot hydrocast in the greater part of facilities in a part of facilities in the composite sample or the morning and the afternoon of 24h though it is an equiponderance mixture of each spot hydrocast that collects once. 1-3 is required while refrigerated from the sampling to 348 Appendix 4Investigation of actual conditions of influent organic matter characterization the measuring water quality because sending out the obtaining water and the sample was requested from the person in charge of the maintenance in the greater part of facilities. Appendix 4Investigation of actual conditions of influent organic matter characterization 349 50 40 30 Number of parts 20 10 0 ~5,000 5,000~ 10,000 10,000~ 50,000 50,000~ 100,000 100,000~ 3 Existing..facilities..ability. /d] Figure A4. 1Distribution of existing process capability of object facilities of relation investigation of CODCr and other carbon-estimating parameters (3)Finding Difference between facilities The relation between BOD5 of influent and CODMn and CODCrFigure A4. 2、Figure A4. 3The cumulative frequency distribution of [ni], the CODCr /BOD5 ratio, and the CODCr /CODMn ratioFigure A4. 4[Ni] was shown. Moreover, amount of statistics according to the CODCr /BOD5 ratio and the CODCr /CODMn ratioTable A4. 1[Ni] was brought together. ・ An excellent correlation is seen between CODCr and BOD5・CODMn of influent. Each average, and the CODCr /BOD5 ratio and the CODCr /CODMn ratio are 2.1, and 3.6. ・ It doesn't depend on the distinction between the facilities scale and the crude sewage and [go] ..sinking.. water and an excellent linear relationship is seen between CODCr and CODMn especially. ・ The crude sewage is CODCr compared with [shizugomizu] in case of BOD5/ It tends ..large the BOD5 ratio... This seems by the crude sewage it is because 350 Appendix 4Investigation of actual conditions of influent organic matter characterization comparatively a lot of small organisms at the use speed by the living being are contained. Appendix 4Investigation of actual conditions of influent organic matter characterization 800 Crude sewage [Shizugomizu] 600 [mg/l] Linear regression Cr 400 COD y = 1.9755x R2 = 0.8222 200 0 0 100 200 300 400 BOD [mg/l] 5 Figure A4. 2Relation between BOD5 and CODCr of influent 800 Crude sewage [Shizugomizu] 600 [mg/l] Linear regression y = 3.6901x R2 = 0.9546 COD400 Cr 200 0 0 50 100 COD Mn 150 200 [mg/l] Figure A4. 3Relation between CODMn and CODCr of influent 351 352 Appendix 4Investigation of actual conditions of influent organic matter characterization 100 80 Cumulative 60 frequency % 40 20 CODCr/BOD CODCr/CODMn 0 0 1 2 3 4 5 6 ratio [-] Figure A4. 4Cumulative frequency distribution of CODCr /BOD5 ratio and CODCr /CODMn ratio of influent Table A4. 1Amount of statistics according to CODCr /BOD5 ratio and CODCr /CODMn ratio of influent Standard Coefficient Number Average Maximum Minimum deviation of of data [-] [-] [-] [-] variation [%] CODCr /BOD5 90 2.05 3.02 1.29 0.34 16.4 ratio CODCr /CODMn 90 3.63 4.77 2.67 0.34 9.4 ratio Appendix 4Investigation of actual conditions of influent organic matter characterization 353 Change in the same facilities The cumulative frequency distribution of the CODCr /BOD5 ratio and the CODCr /CODMn ratio of 65 facilities where the obtaining water of one time was done at each season every seasonFigure A4. 5、Figure A4. 6[Ni] was shown. ・ As for the CODCr /BOD5 ratio, a strong tendency is seen a little in the data of summer (July-August). Moreover, the small tendency is seen a little in the data in winter (January-February) as for the CODCr /CODMn ratio. However, neither is the one that is bigger than the difference between waste treatment facilities. 100 80 Cumulative 60 January-February 40 April-May July-August 20 October-November 0 0 1 2 3 4 5 6 COD Cr/BOD 5 Ratio- Figure A4. 5Cumulative frequency distribution of ratio of every season of influent CODCr /BOD5 354 Appendix 4Investigation of actual conditions of influent organic matter characterization 100 January-February 80 April-May July-August Cumulative frequency % 60 October-November 40 20 0 0 1 2 3 4 5 6 COD Cr/COD Mn Ratio- Figure A4. 6Cumulative frequency distribution of ratio of every season of influent CODCr /CODMn Appendix 4Investigation of actual conditions of influent organic matter characterization 355 Moreover, eight facilities ..adoption.. (..there were comparatively a lot of [mizu] frequencies... Table A4. 2Amount of statistics of) according to the CODCr /BOD5 ratio and the CODCr /CODMn ratio of each facilities 356 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 3、Table A4. 4[Ni] was brought together. ・ The average level of the size of the change of the CODCr /BOD5 ratio and the CODCr /CODMn ratio in the same facilities between the above-mentioned facilities as long as it compares it by the coefficient of variation is equal to the difference. ・ The CODCr /CODMn ratio tends as well as the difference between facilities and the coefficient of variation tends to the small. ・ The clear trend is not seen in the difference of the coefficient of variation value of the crude sewage and [shizugomizu] in three facilities (a,b,e) where both the crude sewage and [shizugomizu] were measured in the same facilities (The coefficient of variation of the crude sewage is not necessarily larger). Table A4. 2Outline of change research objective facilities of CODCr /BOD5 ratio and CODCr /CODMn ratio of influent Existing process Facilities Exclusion scheme Investigation period capability * name [m3/d] Branch a 790,000 2002.8~2004.3 (confluence of b 160,000 Branch 2000.7~2001.3 part) - c 99,000 Branch 2000.7~2001.3 d 28,000 2003.5~2004.3 e 26,000 Branch (confluence of part) Branch f 2,800 Branch 2000.7~2001.3 g 2,600 Branch 2003.5~2004.3 h 1,600 Branch 2000.6~2001.3 Official process capability when investigation is executed. 2002.8~2004.3 Appendix 4Investigation of actual conditions of influent organic matter characterization 357 Table A4. 3Change situation of CODCr /BOD5 ratio of influent Facilitie s name a b c d e f g h Influent Type Data Number Average [-] Maximum [-] Minimum [-] Standard deviation [-] Coefficient of variation Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage Crude sewage Crude sewage 13 2.34 4.03 0.96 0.760 32.5% 16 2.42 3.57 0.98 0.614 25.4% 9 2.12 2.50 1.63 0.271 12.8% 7 1.91 2.20 1.58 0.194 10.1% 9 2.27 3.12 1.49 0.532 23.5% 11 2.69 4.20 2.17 0.619 23.0% 12 2.40 3.16 0.96 0.577 24.1% 14 2.38 3.44 0.99 0.567 23.9% 12 1.85 2.46 1.33 0.317 17.2% 11 3.46 4.36 3.00 0.526 15.2% 16 2.14 2.81 1.54 0.319 14.9% Table A4. 4Change situation of CODCr /CODMn ratio of influent Facilitie s name a b c d e f g h Influent Type Data Number Average [-] Maximum [-] Minimum [-] Standard deviation [-] Coefficient of variation Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage [Shizugo mizu] Crude sewage Crude sewage Crude sewage 15 3.36 3.88 1.68 0.494 14.7% 17 3.43 3.85 2.74 0.274 8.0% 9 3.58 4.04 3.33 0.217 6.1% 8 3.03 3.52 1.58 0.599 19.8% 9 3.85 4.56 3.26 0.364 9.5% 10 3.21 3.94 2.47 0.429 13.4% 12 3.56 3.96 2.98 0.267 7.5% 12 3.45 3.90 3.10 0.211 6.1% 13 2.69 3.51 1.89 0.415 15.4% 11 3.22 3.66 2.75 0.285 8.8% 16 3.54 5.35 2.64 0.661 18.7% 358 Appendix 4Investigation of actual conditions of influent organic matter characterization 3.Investigation of actual conditions of organism element compositionError! Reference source not found. (1)Purpose The segmentation operation based on the OUR measurement was presumed for the influent of 16 facilities with a different condition of presence in the facilities scale and the primary sedimentation tank etc. aiming to understand what organism element composition the influent of the sewage plant in our country had, and very it varied between the treatments and the doing each organism element density was presumed. Additionally, it is a method of STOWA as the representative of a simple estimation method. 4S)The water quality data to do the [niyoru] segmentation collected also, too and the comparison of the estimated results was attempted. (2)Search procedure Object facilities (intended for 16 places of the Kanto region in the sewage plant. Table A4. 5)。When facilities were selected, it was noted that facilities of various scales were covered. The range of an existing process capability of the object facilities (The investigation is executed) is 1,000-910,000 It is m3/d. Table A4. 5Outline of facilities of organism element composition of influent for investigation of actual conditions Name Existing process capability [m3/d] Exclusion scheme Processing mode The first precipitation ground A 1,400 Branch OD method It is not. B 1,000 Branch OD method It is not. C 1,750 Branch OD method It is not. D 3,150 Branch OD method It is not. E 8,250 Branch F 26,060 Branch G 21,200 Branch H 29,200 Branch (confluence of part) I 39,400 Branch J 39,600 K 78,000 Branch (confluence of part) Confluence (part of sub-culture) Standard procedure Standard procedure Standard procedure Standard procedure Standard procedure Standard procedure Standard procedure It is. It is. It is. It is. It is. It is. It is. Appendix 4Investigation of actual conditions of influent organic matter characterization L 195,000 Branch (confluence of part) M 520,000 Branch N 705,000 Confluence O 790,000 Branch (confluence of part) P 910,000 Confluence Standard procedure Standard procedure/A2O Standard method procedure Standard procedure Standard procedure It is. It is. It is. It is. It is. 359 360 Appendix 4Investigation of actual conditions of influent organic matter characterization Sampling and measuring method Two investigations in winter (January-March) of 2003 and spring (May-June) were executed. Influent, the treated water (final sedimentation pond overflow), and the sending back dirt were collected in each facilities, and it supplied it to the experiment operation and the measuring water quality for the presumption of organism element (SA ,SF ,SI ,XS ,XI ,XH ) density in influent. The sedimentation pond overflow ([go] ..sinking.. water) was collected first in the treatment influent (crude sewage) and other facilities in facilities A-D that did not have the primary sedimentation tank for the reactive tank influent as influent it. However, both the crude sewage and [shizugomizu] facilities F and facilities O were collected. The sample is all the spot hydrocasts that collected at 8:30-9:00. The segmentation of the organism element is "Error! Reference source not found. Error! Reference source not found.Though it executed by an experimental estimation method using the OUR measurement introduces by" because XS obviously becomes undervaluation (XI overvalues it as a result) when the crude sewage is targeted in this methodError! Reference source not found.、The estimated result using ultimate BOD based on the method of STOWA was adopted about the life drainage sample. (3)Finding Estimated result of each element Each element density of influent in two investigationsFigure A4. 7[Ni] and the element composition rate (abundance ratio that occupies it to total CODCr)Figure A4. 8[Ni] was shown. Moreover,Table A4. 1Basic amount of statistics concerning the abundance ratio of [niha] and each element was arranged to the crude sewage and [shizugomizuwaka]. ・ Total CODCr varies between facilities, and the coefficient of variation is 20% in the crude sewage, and 28% in [shizugomizu]. The proportion of total CODCr that dissolubility CODCr(S-CODCr) it occupies is 0.45 μm or 0.10 (..39.0% of 26.0 on the average respectively it in the crude sewage when the membrane filter of μm is used 20.7%, and [go] water and ..sinking.. 46.5 on the average respectively it it... Figure A4. 9)。 ・ Crude sewage..average..average..presume..abundance ratio..respectively..average..small.Figure A4. 10)。The difference such as there is the one to exceed 10% by the abundance ratio, too is large while the density and the abundance ratio are greatly different according to facilities, and there is a sample Appendix 4Investigation of actual conditions of influent organic matter characterization 361 not detected at all. ..SA of elements of two that composes SS.. (in the subject ..between this element and SS.. ..seeing a strong positive correlation relation... Figure A4. 11)。On the other hand, (..between S-CODCr, BOD5, and SS.. ..not seeing clear relation..Figure A4. 12、Figure A4. 13)。(..showing a high SS density.. ..inside-large scale.. ..between the length of the pipe and drain and the SS density.. ..not seeing the clear relation.. though it is often facilities, and the time of flow of drainage in the pipe and drain is suggesting the possibility of influencing it in the SS density. Figure A4. 14)。It seems that it is necessary to temper with the factor affecting after the drainage of the contribution of the operation condition and the sludge disposal return-into water in pump [**] and the primary sedimentation tank etc. flows in the waste treatment facility. 800 700 600 XI 500 [mgCOD/L] XH Xs 400 Ss 300 SI 200 100 0 A B C D E F F' G H I J K Treatment name (a) Investigation in winter L M N O O' P 362 Appendix 4Investigation of actual conditions of influent organic matter characterization 800 700 600 XI 500 [mgCOD/L] XH Xs 400 Ss 300 SI 200 100 0 A B C D E F F' G H I J K L M N O O' P Treatment name (b) Investigation in spring Figure A4. 7Influent organism element segmentation result of each waste treatment facility (element density)(A round bawn of the facilities name shows that the crude sewage was targeted). Appendix 4Investigation of actual conditions of influent organic matter characterization 363 100% 80% [%] XI ) Cr T-CO 60% XH Xs Ss 40% ..abundance ratio (.. pair SI 20% 0% A B C D E F F' G H I J K L M N O O' P Treatment name (a) Investigation in winter 100% 80% [%] XI ) Cr T-COD 60% XH Xs Ss 40% ..abundance ratio (.. pair SI 20% 0% A B C D E F F' G H I J K Treatment name (b) Investigation in spring L M N O O' P 364 Appendix 4Investigation of actual conditions of influent organic matter characterization Figure A4. 8Influent organism element segmentation result of each waste treatment facility (element composition ratio)(A round bawn of the facilities name shows that the crude sewage was targeted). Appendix 4Investigation of actual conditions of influent organic matter characterization 365 Table A4. 6Amount of statistics concerning CODCr and the element composition ratio Crude sewage (n=10) Average [-] Index/element Maximum [-] Minimum [-] Standard deviation [-] Coefficient of variation Water quality parameter [mg/L] 492 705 343 97.3 20% S-CODCr, 0.45*1[mg/L] 128 163 102 23.7 18% S-CODCr, 0.10*2[mg/L] 102 140 81 21.5 21% T-CODCr Element abundance ratio of ASM2d SF [-] 0.5 2.5 0.0 0.8 151% SA [-] 2.1 7.5 0.0 2.5 121% SI [-] 3.0 4.5 1.3 1.1 37% XI [-] 15.3 48.2 0.2 14.7 96% XS [-] 70.4 89.8 45.2 12.4 18% XH [-] 8.9 14.4 5.3 2.9 32% [Shizugomizu] (n=24) Average [-] Index/element Maximum [-] Minimum [-] Standard deviation [-] Coefficient of variation Water quality parameter [mg/L] 186 264 74 52.1 28% S-CODCr, 0.45*1[mg/L] 86.4 160 37.0 30.4 35% S-CODCr, 0.10*2[mg/L] 72.6 133 29.0 26.6 37% T-CODCr Element abundance ratio of ASM2d SF [-] 0.8 4.4 0.0 1.2 150% SA [-] 6.3 23.6 0.0 5.2 83% SI [-] 9.8 16.3 5.4 2.7 28% XI [-] 14.0 31.6 2.2 8.8 63% XS [-] 55.2 73.7 37.7 9.8 18% XH [-] 14.4 23.1 7.2 4.8 33% - 1 Pore size 0.45 The filtrate with the membrane filter of μm is measured. - 2 Pore size 0.10 The filtrate with the membrane filter of μm is measured. Appendix 4Investigation of actual conditions of influent organic matter characterization 200 150 Cr 100 S-CO 50 S-CODCr(0.45μm) S-CODCr(0.10μm) 0 0 200 400 T-COD Cr 600 800 [mg/L] (a) Crude sewage 200 S-CODCr(0.45μm) S-CODCr(0.10μm) 150 S-CODCr [mg/L] 366 100 50 0 0 100 200 300 T-CODCr [mg/L] (b) [Shizugomizu] Figure A4. 9Relation between total CODCr and dissolubility CODCr of influent it Appendix 4Investigation of actual conditions of influent organic matter characterization 70 60 Crude sewage [Shizugomizu] 50 [mg/L] 40 SS 30 20 10 0 0 200 400 COD Cr 600 800 [mg/L] Figure A4. 10Relation between CODCr and total SS of influent 80 Crude sewage 60 [Shizugomizu] [mg/L] 40 SS 20 0 0 20 40 60 80 VFA [mgCOD/L] Figure A4. 11Relation between organic acid (SA ) of influent and SS 367 368 Appendix 4Investigation of actual conditions of influent organic matter characterization 70 60 Crude sewage 50 [Shizugomizu] 40 SS 30 20 10 0 0 50 100 S-COD Cr,0.45 150 200 [mg/L] Figure A4. 12Relation between S-CODCr and SS of influent 70 Crude sewage 60 [Shizugomizu] 50 [mg/L] 40 SS 30 20 10 0 0 100 200 300 BOD [mg/L] 5 Figure A4. 13Relation between BOD5 and SS of influent Appendix 4Investigation of actual conditions of influent organic matter characterization 70 60 Crude sewage [Shizugomizu] 50 [mg/L] 40 SS 30 20 10 0 10 100 1000 10000 [km]of soil pipe The total extension Figure A4. 14Pipe and drain (soil pipe) extension and relation of SS of influent 30 Crude sewage 25 [Shizugomizu] 20 [mg/L] 15 SI 10 5 0 0 50 100 S-COD Cr,0.45 150 200 [mg/L] Figure A4. 15Relation between S-CODCr of influent and 0.45 and SI 369 370 Appendix 4Investigation of actual conditions of influent organic matter characterization ・ SI is 8-19 in the crude sewage It is mg/L (14.0 on the average mg/L), [shizugomizu], and 10-27 It is presumed mg/L (17.4 on the average mg/L), and the abundance ratio is 3.0, and 9.8% on each average. SI suggests in small-scale facilities and the inflow load of the processing process suggests that estimate value of this element become small in the small (SRT is long) in the small point comparatively though S-CODCr of the treated water is considered to be SI in the fractionation procedure used. (..S-CODCr of influent in the [shizugo] water sample.. ..little seeing relativity between the two of the life drainage sample.. though SI by high tends to rise. Figure A4. 15)。The coefficient of variation of the abundance ratio to total CODCr is a level that is 37 and 28% respectively a little higher than the coefficient of variation of total CODCr as for the crude sewage and [shizugomizu]. ・ XI is 1-280 in the crude sewage It is mg/L (80.7 on the average mg/L), [shizugomizu], and 3-81 It is presumed mg/L (28.3 on the average mg/L), and the abundance ratio is 15.3, and 14.0% on each average. Both do not have a big difference in the abundance ratio though the crude sewage is higher when seeing in the density. Note the point where presumption is extremely uncertain only because this element is indirectly calculated as a balance with the sum total of total CODCr and other element densities in the fractionation procedure used. It is usual in an actual simulation to adjust this element at the stage of the calibration on the basis of the amount etc. of the excess sludge solid as described in "Business use of the Chapter 4 activated sludge model". ・ XS was 263-511 in the crude sewage It was mg/L (343 on the average mg/L), [shizugomizu], and 45-149 It was presumed mg/L (100 on the average mg/L). It is the maximum constituent in all samples, and the abundance ratio reaches 70.4 and 55.2% on each average. It can be said that both levels of the difference between facilities are the same levels in both as total CODCr in the coefficient of variation of the abundance ratio by 18%. Because the positive correlation is seen between total CODCr though it varies widely, (Figure A4. 16It is possible to presume from total CODCr simply by using an average abundance ratio if it thinks this element density along with above-mentioned XI is adjusted in the calibration. Similar..presumption..floatage..subtract..one..suspended solid..do..think..main enumeration..result..range..index..with..presumption..reliability..improve.Figure A4. 17、Figure A4. 18)。 ・ XH was 31-60 in the crude sewage It was mg/L (42 on the average mg/L), [shizugomizu], and 11-45 It was presumed mg/L (26 on the average mg/L). ..magnitude correlation of 8.9, each 14.4% on the average, and both.. (..the reversal.. when seeing by the abundance ratio though the crude sewage where the concentration of suspended solids is high is obviously more high density. Figure A4. 19) 。 The rejection ratio of the biomass in the primary Appendix 4Investigation of actual conditions of influent organic matter characterization 371 sedimentation tank suggests the small from other organisms in this. The coefficient of variation of the abundance ratio of XH is a level that is 32 and 33% respectively a little larger than total CODCr as for the crude sewage and [shizugomizu]. 372 Appendix 4Investigation of actual conditions of influent organic matter characterization 600 Crude sewage 500 [Shizugomizu] 400 [mg/L] 300 XS 200 100 0 0 200 400 T-COD Cr 600 800 [mg/L] Figure A4. 16Relation between CODCr and total XS of influent 600 Crude sewage 500 [Shizugomizu] 400 [mg/L] 300 XS 200 100 0 0 200 400 600 P-COD [mg/L] Cr Figure A4. 17Relation between floatage CODCr (P-CODCr ) of influent and XS Appendix 4Investigation of actual conditions of influent organic matter characterization 600 Crude sewage 500 [Shizugomizu] 400 [mg/L] 300 XS 200 100 0 0 200 400 600 SS [mgSS/L] Figure A4. 18Relation between suspended solid (SS) of influent and XS 70 60 Crude sewage [Shizugomizu] 50 [mg/L] 40 H X 30 20 10 0 0 200 400 T-COD Cr 600 800 [mg/L] Figure A4. 19Relation between CODCr and total XH of influent 373 374 Appendix 4Investigation of actual conditions of influent organic matter characterization Comparison with estimated result by method of STOWA The biggest difference with the method of the fractionation procedure and STOWA used by the main enumeration is in the estimation method of two elements (SS and XS) used by the living being. Then, the one that the result of presuming both elements by the method of STOWA was compared with the above-mentioned estimated result :. Figure A4. 20、Figure A4. 21....going out.. (.. however, the estimation method of XS when the crude sewage is targeted is not shown because it is identical. ) ・ Method..estimate value..crude clause..show..measurement..based sewage..average..average..preceding on..estimated result..compared with..obviously..high.Figure A4. 20)。Both of estimate values of SF will differ greatly in the law because the both hands method uses both measurements of organic acid for SA. ・ The estimated result of XS by the method of STOWA is 39-161 Mg/L (94.2 on the average mg/L), and it is a method based on the OUR measurement and roughly an estimated result of this level. However, it can be said that small estimate value will be given a little , considering the amount so that estimate value of XS by the former may contain the majority of the amount of the organism of the living being in influent (Correspond to XH). ・ About the influence that the difference of the estimated result by the both hands method gives the simulation result, "Error! Reference source not found. Error! Reference source not found.Refer because it introduces the discussion case to". Appendix 4Investigation of actual conditions of influent organic matter characterization 140 120 [mg/L] 100 Law) 80 STOWA 60 ( SS 40 Crude sewage 20 [Shizugomizu] 0 0 10 20 30 40 50 60 70 S ( OURLaw)[mg/L] S Figure A4. 20Comparison of SS estimate values by OU R method and STOWA method 375 376 Appendix 4Investigation of actual conditions of influent organic matter characterization 200 [mg/L]150 Law) STOWA 100 ( XS 50 [Shizugomizu] 0 0 50 100 150 200 X ( OURLaw)[mg/L] S Figure A4. 21Comparison of XS estimate values by OU R method and STOWA method Comparison with other segmentation cases The one that the estimated result that had been obtained by the main enumeration was compared with a domestic and foreign report case Appendix 4Investigation of actual conditions of influent organic matter characterization 377 Table A4. 7..going out.. . ・ The segmentation result is greatly different according to the report case. ・ There is the estimated result of the main enumeration by SS comparing other segmentation cases based on the OUR measurement and are not some big differences on average excluding the small point. ・ SS is comparatively high in the estimated result based on the method of STOWA as well as this finding. 378 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 7Comparison of influent organism element segmentation results Crude sewage Pres umpt ion Meth od OU Doc ume nt 9~13 (12) OU 4G) Abundance ratio % *1 Object T-CODCr [mg/L] SF SA SI XI XS XH One ..main enumeration *.. 343~705 (492) 0~3 (1) 0~8 (2) 1~5 (3) 0~48 (15) 45~90 (70) 5~14 (9) Sewage plant (Japan; three places) 256~430 (338) 1~4 (2) 0~8 (3) 3~6 (4) 2~3 (3) 73~76 (75) Two ..sewage plant *.. (Turkey; one place) Sewage plant (France; seven places) Sewage plant (Netherlands; 21 places) 590 9 -- 3 11 80 - OU 484~965 (663) 0~13 (5) -- 3 30~63 (47) 24~49 (38) 6~17 (10) OU 241~827 (551) 11~42 (27) 4~10 (6) 23~50 (39) 10~47 (28) - ST Pres umpt ion Meth od OU Err or! Ref ere nce 4K) sou rce not 4S) fou nd. [Shizugomizu] Main enumeration ASM2d technical Report (illustration value) Sewage plant (Japan; six samples a place) [mg/L] SF SA SI XI XS XH 74~264 (186) 0~4 (1) 0~24 (6) 5~16 (10) 2~32 (14) 38~74 (55) 7~23 (14) 260 12 8 12 10 48 12 ー 150~210 2~5 0~15 40~65 6~10 OU 133/186 5/4 0/2 9/4 20/16 54/72 11/3 OU 7~13 (10) 9~24 (14) 5~11 (8) 14~40 (27) 23~34 (29) 9~18 (12) OU 9~35 (23) 9~24 (14) 5~10 (8) 13~19 (17) 24~52 (39) - ST 4K) one 320 14 9 8 47 22 OU three 250~430 (335) 7~11 (9) 10~20 (14) 8~10 (9) 53~60 (56) 7~15 (12) OU 4O) 37 6 10 45 - OU 39 6 24 31 - ST Sewage plant (Japan; five samples a place) Sewage plant (Netherlands; place) one 120~190 (156) 579 4O) - 1 The upper row: In the range and the the lower parentheses: The mean. - 2 Mean of 18 samples. - 3 It is included in XI. 16~32 Doc ume nt Err or! Ref ere nce sou 4G) rce not fou nd. 4T) Sewage plant (Japan; one place) Sewage plant (Switzerland; place) Sewage plant (Switzerland; places) Abundance ratio % *1 T-CODCr Object Appendix 4Investigation of actual conditions of influent organic matter characterization 379 4.Change situation investigation of organism element compositionError! Reference source not found. (1)Purpose In the discussion about propodus, it was clarified that the organism element composition of influent was greatly different according to the sewage plant. On the other hand, it is useful to discuss whether to only have to collect the influent quality data used by the simulation at the frequency of which extent and to set it to understand how the organism element composition changes timewise when the same facilities are seen. When the simulation intended for a season different from the data especially used by the calibration and the simulation of a long term that exceeds one month are done, this becomes an important viewpoint. 4K)。 The influent organism element was measured from such an aspect with three different Thymus vulgaris span (change in the time varying and the week and secular fluctuation) in a real sewage plant, and the change situation was investigated. (2)Search procedure Object facilities Four places in the sewage plant where the process capability was different were targeted (① ..facilities.. -④). The outline of each facilitiesTable A4. 8It is as [ni] is brought together. Table A4. 8Outline of change situation research objective facilities of influent organism element Existing process capability Exclusion scheme One ..process ing mode *.. Primary sedimentation Twotank ..sludge disposal *.. [Mizu] method *3 of adoption Facilities ① Facilities ② Facilities ③ Facilities ④ 2,600 m3/d 26,000 m3/d 28,000 m3/d 790,000 m3/d Branch Branch Branch (confluence of part) OD method A2O method It is not. Standard procedure It is. It is. Branch (confluence of part) Standard procedure It is. Thickness and escape C Thickness and escape of ..erasing.. S Thickness and escape C Thickness, escape, and scorch S - 1 OD method: Oxidation ditch method and standard procedure: Conventional activated sludge process and A2O method: Conceited reluctance-hypoxia-method. - 2 Thickness: Concentrate and/anaerobic digestion and ..erasing.. escape: Dehydration and scorch: Incineration. - 3 [Mizu] method of adoption in main enumeration. C: Composite (24h) and S: Spot (nine o'clock). 380 Appendix 4Investigation of actual conditions of influent organic matter characterization Appendix 4Investigation of actual conditions of influent organic matter characterization 381 Sampling and measuring method Facilities..moon..frequency..obtaining water..do..reaction..tank..influent..facilities..screen..pass..crude sewage..facilities..primary sedimentation tank..overflow..as follows..only..influent..record..for..organism..element..density..understand..segmentation ..operate..secular fluctuation..investigation.The expert day investigation was executed in winter in -③, and ..adoption.. influent that did [mizu] was supplied to the segmentation operation similarly at the regular time (4h interval level) in a day ..facilities ① moreover.. (time varying investigation). In addition, the investigation that examined the influent quality change of one week at the time varying investigation and the simulataneous period was done in facilities ③(change investigation in the week), too. It is a secular fluctuation, a change investigation in the week, and the influent used is a spot ..adoption.. sample in composite sample of 24h that uses autosampler and facilities ② and ④ ..facilities ① and ③.. in about 9:00 that does [mizu] in the morning. The segmentation operation executed by each investigation is a method mainly composed of the OUR measurement similar to propodus. However, only facilities ① intended for the crude sewage used ultimate BOD and XS was presumed. (3)Finding Time varying situation The time varying situation of the influent organism element density in facilities ① -③ Figure A4. 22[Ni] was shown. Moreover, amounts of statistics of the mean, the standard deviation, and the coefficient of variation, etc. of the abundance ratio of each element to total CODCr 382 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 9[Ni] was brought together. ・ The tendency to which total CODCr decreases from midnight to the morning is seen in each facilities. It is similar to a general water quality variability pattern of drainage mainly composed of the domestic wastewater. ・ Each organism element changes timewise about not only the density but also the abundance ratio. Especially, the change of SF and SA is large. For instance, it is 30 in facilities ③ where both element density was comparatively high in the sample at 13 o'clock and 21 o'clock by gross weight (SS ) of both It is hardly detected in the sample at 1 o'clock and five o'clock of midnight while mg/L is exceeded. ・ The point that the density of SA of [shizugomizu] changes timewise and greatly is a case where the continuous monitoring with the automatic measurement device of organic acid is executed. Error! Reference source not found. However, it is shown. It is considered that the deposit's to which it rotted when the relation is seen in the variability pattern of the pump discharge and the organic acid concentration, and the pump discharge is increased and the flow rate in the incurrent canal rises in the pipe and drain flowing in contributes there. Appendix 4Investigation of actual conditions of influent organic matter characterization 350 300 XI 250 mg/L] 200 [ XH Xs 150 Ss 100 SI 50 0 9:00 13:00 17:00 21:00 1:00 5:00 9:00 Time (a) Facilities ① (H15.12.9-10) 300 250 XI 200 mg/L] [ XH 150 Xs Ss 100 SI 50 0 10:00 12:00 14:00 18:00 22:00 2:00 6:00 Time (b) Facilities ② (H16.3.2-3) 300 250 XI 200 mg/L] XH 150 Xs [ Ss 100 SI 50 0 9:00 13:00 17:00 21:00 1:00 5:00 9:00 Time (c) Facilities ③ (H16.1.28-29) Figure A4. 22Time varying of influent organism element density 383 384 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 9Amount of statistics concerning organism element abundance ratio in time varying investigation Facilitie s ① Facilitie s ② Facilitie s ③ Abundance ratio % T-CODCr [mg/L] SF SA SI XI XS XH Average 231 0.4 0.0 5.1 23 60 12 Maximum 304 1.3 0.0 9.8 29 70 18 Minimum 110 0.0 0.0 3.5 16 52 8.9 Standard deviation Coefficie nt of Average variation 69 0.5 - 2.2 6.0 6.1 3.1 30% 110% - 43% 26% 10% 27% 235 1.6 0.0 6.4 17 62 14 Maximum 280 3.7 0.0 9.6 19 65 18 Minimum 167 0.0 0.0 5.1 14 56 10 Standard deviation Coefficie nt of Average variation 42 1.2 - 1.7 2.1 3.8 2.8 18% 75% - 26% 13% 6% 21% 206 2.9 4.8 8.0 14 53 17 Maximum 266 4.9 11 9.8 21 57 22 Minimum 154 0.0 0.0 5.9 6.2 49 12 Standard deviation Coefficie nt of variation 44 2.1 4.4 1.4 4.9 2.8 4.1 21% 71% 91% 18% 35% 5% 24% (..examination of time varying situation in the following three places in influx route in waste treatment facility as index of organic acid (SA ) to clarify variation factor of SS of influent in facilities ③..Figure A4. 23)。 ‒ Part A: The upstream part of inflow screen (crude sewage before sludge disposal return-into water mixes) ‒ Part B: The first sedimentation pond inflow part (crude sewage after return-into water mixes) ‒ Part C: Reactive tank inflow part (the first sedimentation pond overflow) ・ The density is overall small, and time variance's pattern is also greatly different from another though the time variance to whom SA is roughly similar is shown in part B and C in part A. ・ The composition of SA is different part only A. As for things except the detection of the propionate in part A with the sample at 17 o'clock while the propionate and the butyrate were detected in part B・C at time zone with high density of SA, only the acetate has been detected. Appendix 4Investigation of actual conditions of influent organic matter characterization 385 ・ It can be guessed to the time varying of the density of SA seen in the reactive tank influent of this facilities that the generation of SA with SA and inflow pump [**] of the sludge disposal return-into water origin contributes greatly from the change of the influent quality to the waste treatment facility from these. Actually, the case where high density organic acid is detected with the gravity concentrate tank overflow and the dehydration separation liquid is reported. not found., Error! Reference source not found. 。 Error! Reference source 386 Appendix 4Investigation of actual conditions of influent organic matter characterization 60 Crude sewage VFA50 [mgCOD/L] In front of [shizu] naive 40 [Shizugomizu] 30 20 10 0 9:00 13:00 17:00 21:00 1:00 5:00 9:00 Time Crude sewage:In front of [shizu] naive of the style (There is not a return-into water mixing) ..on the inflow screen..: the first [go] ..sinking.. water of the sedimentation pond influent (After the return-into water mixes): Reactor influent. Figure A4. 23Time varying of organic acid concentration in inflow system each place (Facilities ③; H16.1.28-29) 387 Appendix 4Investigation of actual conditions of influent organic matter characterization Change situation in week The result of the change investigation in the week executed in facilities ③Figure A4. 24And,Table A4. 10[Ni] was shown. ・ Total CODCr and each organism element abundance ratio are steady though the average water quality of the day based on the composite sample is used here compared with the above-mentioned time varying. There is no rainfall for this investigation period. ・ However, the change of SF and SA day in and day out is large, and the coefficient of variation equals 1.20 respectively, 0.37, a time varying, and the following secular fluctuations. ・ The difference of the influent quality by the presence of dehydration is not clear though the dehydration work is not done in this facilities on the soil and Sunday. 300 [mg/L] 250 XI 200 XH 150 Xs 100 Ss SI 50 0 1/27 1/28 1/29 1/30 (Tue) (Wed) (Thu) (Fri) 1/31 2/1 2/2 2/3 (Sat) (Sun) (Mon) (Tue) Date Figure A4. 24Change in week of influent organism element density (Facilities ③; H16.1.27-2.3) Table A4. 10Amount of statistics concerning organism element abundance ratio in change investigation in week Facilitie s ③ Abundance ratio % T-CODCr [mg/L] SF SA SI XI XS XH Average 199 2.2 1.8 7.6 15 55 18 Maximum 215 3.6 6.2 7.9 22 63 20 Minimum 182 1.2 0.0 7.4 7.6 50 15 Standard deviation Coefficie nt of variation 12 0.8 2.1 0.2 4.4 4.1 1.6 0.06 37% 120% 2% 29% 7% 9% 388 Appendix 4Investigation of actual conditions of influent organic matter characterization Long-term change situation The result of the long-term change investigation in facilities ① -④Figure A4. 25And, 389 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 11[Ni] was shown. ・ The tendency with a large change of SF and SA is seen in each facilities compared with total CODCr and another element as well as the change in the time varying and the week. ・ The tendency to a seasonal change is not clear. However, the tendency to which the density and the abundance ratio of SF ・SA become small in winter is seen in facilities ① -③. Especially, both elements are not detected with the greater part of samples in facilities ① and ② at one February-March. ・ In facilities ④ including the confluence district, the tendency to which total CODCr extremely becomes a low concentration at the rainfall, and the abundance ratio of SF and SA becomes small in addition in that is seen. This not only is diluted drainage at the rainfall but also suggests that conversion in the inflow process including the incurrent canal ditch be greatly influenced. However, the influence of the rainfall doesn't appear clearly similarly in facilities ③ including 300 250 250 XI 200 mg/L] [ XH 150 Xs Ss 100 Rainfall Rainfall → 300 → the confluence district. XI 200 mg/L] [ XH 150 Xs Ss 100 SI 50 SI 50 0 0 5/28 7/9 9/30 10/5 10/29 12/9 Date (a) Facilities ① 1/28 2/25 3/24 4/30 5/20 6/24 8/4 9/11 11/25 12/9 1/14 2/24 Date (b) Facilities ② 3/2 390 Rainfall 300 250 XI 200 mg/L] XH 150 Xs [ Rainfall → 350 → Rainfall → Rainfall Rainfall → → 300 Appendix 4Investigation of actual conditions of influent organic matter characterization Ss 100 SI 50 XI 250 mg/L] 200 [ XH Xs 150 Ss 100 SI 50 0 0 5/29 6/26 7/30 8/27 9/17 10/3011/27 12/18 1/28 2/26 3/25 Date (c) Facilities ③ 3/13 5/1 6/18 8/6 9/2 11/17 12/16 1/15 2/27 3/31 Date (d) Facilities ④ Figure A4. 25Secular fluctuation of influent organism element density 391 Appendix 4Investigation of actual conditions of influent organic matter characterization Table A4. 11Amount of statistics concerning organism element abundance ratio in long-term change investigation Facilitie s ① Facilitie s ② Facilitie s ③ Facilitie s ④ Abundance ratio % T-CODCr [mg/L] SF SA SI XI XS XH Average 225 0.5 0.6 4.8 18 64 13 Maximum 284 1.3 1.5 6.1 30 76 15 Minimum 158 0.0 0.0 3.7 2.2 55 10 Standard deviation Coefficie nt of Average variation 47 0.4 0.6 1.0 7.8 6.6 1.6 21% 96% 108% 20% 44% 10% 12% 186 2.1 3.6 9.1 19 49 17 Maximum 246 5.6 10 15 35 58 20 Minimum 136 0.8 0.0 5.9 7.7 42 14 Standard deviation Coefficie nt of Average variation 40 1.5 4.2 2.6 8.0 6.5 2.2 22% 72% 116% 29% 42% 13% 13% 193 2.3 5.1 8.4 14 51 19 Maximum 226 6.5 13 10 31 68 23 Minimum 163 0.0 0.0 7.0 2.8 28 15 Standard deviation Coefficie nt of Average variation 20 2.2 5.0 1.1 9.9 12 2.7 11% 95% 98% 12% 69% 24% 14% 224 1.4 8.0 9.4 20 46 16 Maximum 305 3.6 13 13 27 53 22 Minimum 91 0.0 0.0 6.9 13 41 12 Standard deviation Coefficie nt of variation 68 1.3 4.3 2.1 4.6 3.9 2.7 30% 94% 54% 23% 24% 8% 17% Bibliography 4A) Green willow Hiroshi, the Ono faith, Naoki Mikuni, and [bin;yutaka] Hamada: About the organism composition of influent and the relation of the denitrification, the 39th drainage research symposium lecture collection and pp.632-634,2002. 4B) Hiroshi Itokawa [**], Toshikazu Hashimoto, and Hitoshi Nakazawa: Investigation of actual conditions of sewage plant influent organic constituent for activated sludge model, the 37th aqueous environment society annual meeting lecture collection, and pp.171,2003. 4C) Hiroshi Itokawa [**], Toshikazu Hashimoto, and Takao Murakami: Segmentation, the 40th drainage research symposium lecture collection, and pp.162-164,2003 of sewage plant influent organic constituent that assumes use by activated sludge model. 4D) Hiroshi Itokawa [**], Toshikazu Hashimoto, and Takao Murakami: Measurement of segmentation of organic constituent in drainage for activated sludge model, environmental 392 Appendix 4Investigation of actual conditions of influent organic matter characterization engineering study collection, Vol.40, and pp.41-52,2003. 4E) Hiroshi Itokawa [**] and Takao Murakami: The change realities in sewage plant at influent organic fraction, the 41st drainage research symposium lecture collection, and pp.101-103,2004. Appendix 4Investigation of actual conditions of influent organic matter characterization 393 4F) Hiroshi Itokawa [**] and Takao Murakami: The change realities of influent organic constituent, and influence, environmental engineering study collection, Vol.41, and pp.547-557,2004 in real sewage plant that it gives to activated sludge model simulation 4G) Masami Ebisawa, Kitamura lucidity, Toshio Yoshida, and north [okukiyogyou]: Influence on behavior of organic acid concentration in confluence type sewage plant and phosphorus removal, the 40th drainage research symposium lecture collections, and pp.736-738,2003. 4H) Mieko Kuwana, Koji Takahashi, and pure [**] Tsukahara: The 41st drainage research symposium lecture collection and pp.110-112,2004 discussed concerning method of activated sludge model of organism segmentation (the 2). 4I) Takao Shuto, [miyatajun], Satoshi Ohashi 1, Hiroshi Itokawa [**], Toshikazu Hashimoto, and Takao Murakami: Construction, the 40th drainage research symposium lecture collection, and pp.189-191,2003 of process model that assumes design aid of oxidation ditch method facilities. 4J) [Yutakaki] Narita, [funemizunaogyou], and high [kuwatetsuo]: Research, environmental engineering study collection, Vol.39, and pp.267-278,2002 to nutrient removal of sludge disposal return-into water concerning effective use strategy. 4K) Ahnert,M., Kuehn,V. and Krebs,P.: Identification of overall degradation in sewer systems from long-term measurements and consequences for WWTP simulations. Wat.Sci.Tech., Vol.52, No.3, pp.153-161, 2005. 4L) Funamizu,N., Yamamoto,S., Kitagawa,Y. and Takakuwa,T.: Simulation of the operational conditions of the full-scale municipal wastewater treatment plant to improve the performance of nutrient removal. Wat.Sci.Tech., Vol.36, No.12, pp.9-18, 1997. 4M) Ginestet,P., Maisonnier,A. and Sperandio,M.: Wastewater COD characterization: Biodegradability of physico-chemical fractions. Wat.Sci.Tech., Vol.45, No.6, pp.89-97, 2002. 4N) Itokawa,H., Hashimoto, T. and Murakami,T.: Characterization of influent organic matter for activated sludge modelling in Japan municipal wastewater treatment plants. Proceedings of Asian Waterqual 2003 (CD-ROM), Bangkok, Oct.19-23, 2003. 4O) Jiang,T., Liu,X., Kennedy,M.D., Schippers,J.C. and Vanrolleghem,P.A.: Calibrating a side-stream membrane bioreactor using Activated Sludge Model No.1. Wat.Sci.Tech., Vol.52, No.10-11, pp.359-367, 2005. 4P) Kappeler,J. and Gujer,W.: Estimation of kinetic parameters of heterotrophic biomass under aerobic conditions and characterization of wastewater for activated sludge modelling. Wat.Sci.Tech., Vol.25, No.6, pp.125-139, 1992. 4Q) M?kinia,J. and Wells,S.A.: A general model of the activated sludge reactor with dispersive flow- I. Model development and parameter estimation. Wat.Res., Vol.34, pp. 3987-3996, 2000. 4R) Orhon,D., Cokgor,E.U. and Sozen,S.: Experimental basis for the hydrolysis of slowly biodegradable substrate in different wastewaters. Wat.Sci.Tech., Vol.39, No.1, pp.87-95, 1999. 4S) Roeleveld,P.J. and van Loosdrecht,M.C.M.: Experiences with guidelines for wastewater characterisation in The Netherlands. Wat.Sci.Tech., Vol.45, No.6, pp.77-87, 2002. 394 4T) Appendix 4Investigation of actual conditions of influent organic matter characterization Sollfrank,U. and Gujer,W.: Characterisation of domestic wastewater for mathematical modelling of the activated sludge process. Wat.Sci.Tech., Vol.23, pp.1057-1066, 1991. Appendix 5Comparison of various fractuation method of influent organic matter 395 Appendix5Comparison of various methods of influent organism element segmentation 1.Outline Fixed something doesn't exist though various methods to measure and to presume the amount (density) in influent of each organism element (SA ,SF ,SI ,XS ,XI ,XH ) defined by ASM2d are proposed as described in "Business use of the Chapter 4 activated sludge model". Here, it introduces the result of presuming and comparing each organism element densities by using the water quality and the OUR measurement result of influent in a real sewage plant by eight methods including the influent quality presumption tool built in a simulator on the market in addition to three kinds introduce by this volume. In addition, this facilities are actually simulated by using each influent quality, and it introduces the result of evaluating the influence that the difference of the estimation method gives the simulation result. 2.Review method (1)Object facilities Real facilities of a conceited reluctance-hypoxia-method were targeted. The operating condition when the facilities parameter and the investigation is executed 396 Appendix 5Comparison of various fractuation method of influent organic matter Table A5. 1[Ni] was brought together. (2)Use data The data used to presume each organism elementTable A5. 2[Ni] was brought together. These are based on the water quality data and the OUR measurement result that collected when the expert day executed for the calibration of the model is investigated. However, the mean of 24h was used from the intention to facilitate the comparison of the results for all items. (3)Estimation method of organism element Table A5. 2(..drinking and the use of data.. ..each organism element (SA ,SF ,SI ,XS ,XI ,XH ) according to eight methods.. ..the calculation of the density... Table A5. 3)。 Method ① (OUR) ・・・・・・ In the method mainly composed of the OUR measurement, (a) (b) that divides SS and XS from the difference of the OUR level The point of presuming XH from OUR is a feature. A complex OUR measurement in addition to water analyses of S-CODCr of T-CODCr, organic acid, and the treated water of influent is needed. 397 Appendix 5Comparison of various fractuation method of influent organic matter Processing mode Facilities parameter Capacity reactive tank 2,190 m3 (reluctance: m3×3) Operating condition etc. Capacity of the final sedimentation Influent quantity pond Dirt sending back ratio Internal circulation ratio 960 m3 SRT 12.7d Water temperature reactive tank MLSS Conceited DO - of Table A5. 1Outline of object facilities Conceited reluctance-hypoxia-method sedimentation tank). of 236 m3 and hypoxia: (There is a primary 236 m3×3 and conceit: 415 5,160 m3/d 0.3 1.05 16.2℃ 2,580 mg/L tank The first division: 0.3 mg/L and the 2nd division: 1st division: 1.6 Mg/L 0.6 mg/L and the The operating conditions etc. are the day all ..adoption.. means on [mizu] execution day. Table A5. 2Outline of measurement data used to presume influent quality Water quality item CODCr Influent quality (water analyses) Treated effluent quality - Remarks 192 S-CODCr (0.45 μm) 78 S-CODCr (0.10 μm) 70 Ultimate BOD(BODn) 93 Suspended solid (TSS) 65 VFA Influent quality (OUR measuremen t) Density * [mgCOD/L] Presumption from BOD1, 2, 5, 7, and 12. 7 Refer to "Appendix estimation method. SS 14 XS 109 〃 XH 34 〃 S-CODCr (1 μm) 16 Simple mean of 24 4h pitch × h. 3" for the 398 Appendix 5Comparison of various fractuation method of influent organic matter Method ② (JS#1) ・・・・・・ It is a method for use in JS chiefly, and the SS presumption by the OUR measurement and the combination of the XS presumption by the ultimate BOD measurement are the points. Necessary water analyses are S-CODCr of T-CODCr of influent, organic acid, ultimate BOD, and the treated water. Method ③ (JS#2) It is a simple method that assumes the OUR measurement and the ultimate BOD measurement to be unnecessary to method ② of ・・・・・・, and presumes according to conversion factor (Only about T- CODCr;SS, organic acid) based on investigation of actual conditions by JS instead. Method ④ (STOWA) It is a simple method proposed by STOWA of the ・・・・・・ Netherlands. (b) that doesn't measure OUR, and presumes the gross weight of the organism of a possible biodegradation from the (a) ultimate BOD measurement result (c) into which SS and XS are physically divided by furnace paper (0.10 μm) It has the feature that XH doesn't expect it. Measurements of S-CODCr of T-CODCr of influent, organic acid, ultimate BOD, and the treated water are used. Method ⑤ (SIM#1) It is a simple method built in the simulator of the ・・・・・・ marketing. A floating element and a dissolubility element are first divided on the basis of gross weight (Σ X) of the floating element presumed from TSS measurements, and each breakdown is calculated according to conversion factor. At least, T-CODCr of influent and the measurement data of TSS are necessary. Method ⑥ (SIM#2) In the simple method built in the simulator of the ・・・・・・ marketing, the point to calculate a floating element and gross weight each dissolubility element first of all on the basis of TSS measurements and the point to calculate the breakdown from conversion factor are similar to method ⑤. At least, T-CODCr of influent and the measurement data of TSS are necessary. Method ⑦ (SIM#3) All elements that exclude XS are calculated from conversion factor to T-CODCr by the simple method built in the simulator of the ・・・・・・ marketing. At least, the measurement data of T-CODCr of influent is necessary. Method ⑧ (default) ・・・・・・ All elements were calculated from T-CODCr on the basis of the influent quality illustrated in the technical report of ASM2d. Appendix 5Comparison of various fractuation method of influent organic matter 399 Note the following points about method ⑤ -⑦ to refer to a simulator on the market. ・ These simple methods are not recommended in each simulator. It handles it as an alternative method when it is not possible to experiment at an enough data collection and the batch. ・ These are basically input data, and the user sets it though the numerical value is given to conversion factor used beforehand. That is, work to decide the coefficient on the basis of the data measured in the object facilities is needed if original. 5A)。 ・ The plural corresponding to data that can be used kind of influent quality setting tool is installed in a simulator on the market usually. It removes from the object, and T-CODCr is equal to the method of presuming T-CODCr of influent in all the methods though the one with an unnecessary measurement of T-CODCr is included in that in this discussion. As the corresponding method when the measurement data of CODCr is not obtained, the method of presuming the gross weight of the organism of a possible biodegradation from BOD5 is a subject (For instance, rate constant kBOD is assumed to be a parameter from BOD5 and ultimate BOD is presumed), and the coefficient value used there should note it similar to on. (4)Simulation methodology The simulation of the object facilities was executed by using the influent quality presumed by the each way. The condition is a simulation by the steady condition that gives the average data of the day when the investigation is executed as follows. ・ The reactive tank composition in the process model was assumed to be real facilities and identical. ・ The final sedimentation pond model was assumed to be an ideal sedimentation pond model who with the same capacity as real facilities. ・ The day mean when everything was investigated on the expert day was used for various flow rate and water temperatures. Dirt pulled out and set the flow rate on the basis of this investigation date Kon's SRT. ・ The day mean when influent qualities (SNH4 ,SNO3 ,SPO4 ,SALK ) other than the organism element were investigated this was used. ・ DO of the conceited tank each division was compulsorily fixed to the day mean when this investigating. ・ All defaul t values were used for the parameter of ASM2d. 400 Appendix 5Comparison of various fractuation method of influent organic matter ・ Numerical calculation by Runge-the fourth Kutta methods (*t=10s). The simulation of each influent quality for 90d was executed, and each element density at that time was used to evaluate the result. Each element density of a reactive tank reached roughly regularly on this calculation condition in all the simulations though initial condition was assumed to be a stationary solution when the estimated result by method ① was used. Table A5. 3Outline of each organism element estimation method Element Method ① (OUR) Method ② (JS#1) SS ・Presumption from OUR measurement result ・Presumption from OUR measurement result ・Conversion from SA (SS =1.1×SA+1.4) ・ S-CODCr and 0.10 μ m measurement value- SI SA ・Organic acid measurement value ・Organic acid measurement value ・Organic acid measurement value ・Organic acid measurement value SF ・SS-SA ・SS-SA ・SS-SA ・SS-SA SI ・ Treated water S-CODCr measurement value XI ・T-CODCr-Σ another element ・T-CODCr-Σ another element ・T-CODCr-Σ another element ・T-CODCr-Σ another element XS ・Presumption from OUR measurement result ・Presumption from ultimate BOD and SS ・T-CODCr×0.55 ・Presumption from ultimate BOD and SS XH ・Presumption from OUR measurement result ・Presumption from OUR measurement result ・T-CODCr×0.15 ・It doesn't expect it. Remark s ・T-CODCr is an actual measurement value. ・T-CODCr is an actual measurement value. ・T-CODCr is an actual measurement value. ・T-CODCr is an actual measurement value. actual ・ Treated water S-CODCr measurement value Method ③ (JS#2) actual ・ Treated water S-CODCr measurement value Method ④ (STOWA) actual ・ Treated water S-CODCr measurement value ×0.9 actual actual Table A5. 3..(.. ..continuation..) Method ⑤ (SIM#1) Element Method ⑥ (SIM#2) Method ⑦ (SIM#3) Method ⑧ (default) SS - - ・ T-CODCr × /T-CODCr ratio) SA ・ΣS × coefficient (SA /ΣS ratio) ・Organic acid measurement value ・SS × coefficient (SA /SS ratio) ・T-CODCr×0.077 SF ・ΣS × coefficient (SF /ΣS ratio) ・ΣS × coefficient (SF /ΣS ratio) ・SS-SA ・T-CODCr×0.115 SI ・ΣS-SA-SF ・ΣS × coefficient (SI /ΣS ratio) ・T-CODCr × coefficient (SI /T-CODCr ratio) ・T-CODCr×0.115 XI ・ΣX-XS-XH ・ΣX-XS-XH ・T-CODCr × coefficient (XI /T-CODCr ratio) ・T-CODCr×0.096 XS ・ΣX × coefficient (XS /ΣX ratio) ・ΣX × coefficient (XS /ΣX ratio) ・T-CODCr-Σ another element ・T-CODCr×0.481 XH ・ΣX × coefficient (XH /ΣX ratio) ・ΣX × coefficient (XH /ΣX ratio) ・ T-CODCr × /T-CODCr ratio) ・ Σ X=TSS ÷ coefficient (TSS/COD ratio) ・ΣS=T-CODCr-ΣX ・T-CODCr is an actual measurement value. ・ Σ X=TSS × coefficient (COD/VSS ratio)× coefficient (VSS/SS ratio) ・ΣS=T-CODCr-ΣX ・T-CODCr is an actual measurement value. ・T-CODCr is an actual measurement value. Remark s coefficient coefficient (SS (XH - ・T-CODCr×0.115 ・T-CODCr is an actual measurement value. Appendix 5Comparison of various fractuation method of influent organic matter 403 3.Result of review (1)Estimated result of organism element The influent organism element density presumed by the each wayFigure A5. 1[Ni] was shown. Because 0 had been given to the initial value only as for SA in method ⑥, the measurement data of organic acid in facilities A was used though the one substituted for each simulator as a initial value was used for conversion factor used by method ⑤ -⑦. ・ Each organism element density is greatly different depending on the estimation method. ・ In the method (① and ②) using the OUR measurement, SF is a small, and the amount XS is large estimated results. ・ Method ③ is presumption based on the investigation of actual conditions result of the sewage plant in our country based on the OUR measurement, and estimate value equal with method ① and ②. ・ In method ④, SF and XS are this almost densities and features the point that XI is presumed remarkably greatly. ・ The point where the abundance ratio of SF is comparatively large is common though a big difference is seen in the estimated result in method ⑤ -⑦ based on a simulator on the market. ・ SS can be divided roughly into a small group ( ① ..method.. - ③ ) and a large group (④ ..method.. -⑥) with SS(SA+SF ) when paying attention to the division of XS. Method ⑦ and method ⑧(It is based on the component ratio illustrated in the technical report of ASM2d) are the intermedius levels of both groups. 404 Appendix 5Comparison of various fractuation method of influent organic matter 200 XI [mgCOD/L] 150 XH XS SF 100 SA SI ⑧default ⑦SIM#3 ⑥SIM#2 ⑤SIM#1 ④STOWA ③JS#2 ②JS#1 0 ①OUR 50 Figure A5. 1Estimated result of influent organism element density by various methods Appendix 5Comparison of various fractuation method of influent organic matter 405 ・ It is not clear whether it is originates in the estimation method that the point that SS becomes a low concentration in method ① -③ uses the OUR measurement or a general feature of the municipal sewage in our country (Method ④ -⑧ uses the coefficient value etc. found in Europe and entire North America). ・ In ASM2d, the organic nitrogen and the phosphorus concentration are calculated from each organism element by conversion factor (iN,i ,iP,i ). (..all the conversion factors.. ..the difference of an organism element concentration estimation value the above-mentioned when the default value is used.. ..becoming also of the calculation value of the organic nitrogen and the phosphorus concentration of influent a big difference and appearance... Figure A5. 2)。 (2)Simulation result that uses various organism element estimate values With the actual measurement value (day mean) when SNH4, SNO3, and the SPO4 density (profile) of the reactive tank each division in the simulation of which the input data is each estimated result are investigated on the expert dayFigure A5. 3~Figure A5. 5[Ni] was shown. Moreover, SNH4, SNO3, and the SPO4 density of the treated water (reactive tank end division)Figure A5. 6Living being element (XH ,XPAO ,XAUT ) and the XTSS density of [ni] and this divisionFigure A5. 7[Ni] was shown. ・ The difference of the influent quality according to the estimation method especially has a big influence on the expectation of the phosphorus removal, and the concentration profile of SPO4 and the simulation result of the amount of XPAO are greatly different depending on the estimation method. ・ In method ① -③, a density of treated water SPO4 that is small SPO4 burst size in the reluctance tank, higher than that of the actual measurement value is given. SF estimate value reflected the small point in this. ・ On the other hand, SPO4 burst size in reluctance-hypoxia tank has risen overall than the actual measurement value though the SPO4 density of the treated water has decreased even at the measurement value level in method ④ -⑧. 406 Appendix 5Comparison of various fractuation method of influent organic matter 8 7 6 5 [mg/L] Org-N 4 Org-P 3 2 1 0 OUR JS#1 JS#2 ① ② ③ STOWA SIM#1 ④ ⑤ SIM#2 SIM#3 default ⑥ ⑦ ⑧ Figure A5. 2Calculation result of organic nitrogen and phosphorus concentration of influent that uses each estimated result Appendix 5Comparison of various fractuation method of influent organic matter 407 ・ The difference between the above-mentioned SPO4 density and the profile is the levels that greatly change the policy of the calibration when thinking the fitting is done to the measurement data shown here in the calibration. For instance, it will be adjusted that the SPO4 density is decreased in method ④ -⑧ while becoming in method ① -③ the adjustment of the direction where the SPO4 density is increased to reproduce the behavior of SPO4 under the anoxic. ・ The XI density of a reactive tank is greatly different according to the difference of the estimated result of XI of influent. That is, the simulation result of the reactive tank solid concentration and the amount of the excess sludge solid is also greatly different depending on the setting method of the influent quality. Bibliography 5A) Makinia,J., Swinarski,M. and Dobiegala,E.: Experiences with computer simulation at two large wastewater treatment plants in northern Poland. Wat.Sci.Tech., Vol.45, No.6, pp.209-218, 2002. 408 Appendix 5Comparison of various fractuation method of influent organic matter Measurement 18 16 14 [mgN/L] 12 10 NH4 8 6 S 4 2 0 ① OUR ② JS#1 ③ JS#2 ④ STOWA ⑤ SIM#1 ⑥ SIM#2 ReluctanceHypoxia 1 Hypoxia 2 Hypoxia 3 Conceit 1 Conceit 2 Conceit 3 Reactive tank division ⑦ SIM#3 ⑧ default Figure A5. 3Reactive tank profile in simulation that uses each estimated result (SNH4 ) 8 Measurement 7 ① OUR 6 [mgN/L] ② JS#1 5 ③ JS#2 4 NO3 S 3 ④ STOWA 2 ⑤ SIM#1 1 ⑥ SIM#2 0 ReluctanceHypoxia 1 Hypoxia 2 Hypoxia 3 Conceit 1 Reactive tank division Conceit 2 Conceit 3 ⑦ SIM#3 ⑧ default Figure A5. 4Reactive tank profile in simulation that uses each estimated result (SNO3 ) 409 Appendix 5Comparison of various fractuation method of influent organic matter Measurement 20 ①We 15 MgP/lambert ②JS#1 ③JS#2 10 PO4 ④STOWA S ⑤SIM#1 5 ⑥SIM#2 0 ReluctanceHypoxia 1 Hypoxia 2 Hypoxia 3 Self-conceitSelf-conceit 1 Self-conceit 2 3 Reactive tank force ⑦SIM#3 ⑧Default Figure A5. 5Reactive tank profile in simulation that uses each estimated result (SPO4 ) 410 Appendix 5Comparison of various fractuation method of influent organic matter 8 7 6 [mg/L] 5 4 3 2 1 0 SNH4 実測 ①OUR ②JS#1 SNO3 ③JS#2 ④STOWA SPO4 ⑤SIM#1 ⑥SIM#2 ⑦SIM#3 ⑧default Figure A5. 6Element density of reactive tank end division in simulation that uses each estimated result (SNH4 ,SNO3 ,SPO4 ) 2,000 1,800 2,670 1,600 [mg/L] 1,400 1,200 1,000 800 600 400 200 XH ①OUR ②JS#1 XPAO ③JS#2 ④STOWA XAUT ⑤SIM#1 ⑥SIM#2 XI ⑦SIM#3 ⑧default Figure A5. 7Element density of reactive tank end division in simulation that uses each estimated result (XH ,XPAO ,XAUT ,XI ) Appendix 6Sensitivity analysis example 411 Appendix6Example of sensitivity analysis 1.Outline Work to evaluate that it is the impact combination of giving of various parameter values in the activated sludge model and the input data, etc. when simulating it to the simulation result is called "Sensitivity analysis". It does defining the condition of becoming basic (case etc. to use the default value for all parameters), and repeating the simulation to which the parameter etc. made a analysis object there are changed, and the calculation result is compared and will be evaluated specifically. In "Business use of the Chapter 4 activated sludge model", this work is not necessarily done with one process of the calibration though locates as indispensable work. However, when the calibration and the simulation result are evaluated as the influence that the uncertainty of (b) process model and input data to be able to extract the candidate of the parameter and the input condition adjusting it at the (a) calibration by the sensitivity analysis gives the simulation result can be understood, useful information can be obtained. Additionally, it can be said that it is one of the very effective methods to understand the meaning and the role of each parameter in the model to consider that it is the impact combination about each parameter value in detail. Here, it introduces the result of doing the sensitivity analysis for the waste treatment facility of virtual, that the system configuration is different four kinds of. It is sure to become useful information as a material to understand the image of the sensitivity analysis work and to consider the meaning of parameter value and other condition settings though this result cannot be applied to other cases as it is because it is different the impact combination about parameter value and the input condition depending on the composition and the condition of the processing system that targets it. Additionally, it introduces the case where the influence of the time varying of the case and the inflow load (volume of water and water quality) in which the influence of parameter value involved in the simulation result of the biological phosphorus removal is discussed more in detail is discussed in the sensitivity analysis. The former is a part of a series of discussion executed to discuss the calibration procedure related to the phosphorus removal. Moreover, the latter was executed as a part that discussed the amount and the quality of appropriate input data for the simulation. 412 Appendix 6Sensitivity analysis example 2.Sensitivity analysis intended for various processing mode (1)Outline Facilities virtual that seems to be typical it of four kinds of activated sludge processes (reluctance ..a conceited method and the circle method nitrification denitrification method..-conceited active sludge process and reluctance-hypoxia-conventional activated sludge process) used are set in the sewage plant in our country. The sensitivity analysis that targeted the item that the user had to set when simulating it like all parameters, the influent qualities, and the operating conditions etc. of ASM2d covering it was executed. (2)Review method Object facilities A parameter of virtual facilities (A~D) made the object of the analysis and a basic operating condition, etc.Table A6. 1[Ni] was brought together. When a calculation of capacitance and various operating conditions are set, an existing design principle. 6A)It referred to [wo]. Process model The process model was constructed on the basis of the set facilities parameter, and the influent quality and the operating condition were set. The main note is as follows. ・ ASM2d was used as a biological response model. ・ A reactive tank was expressed according to the complete mixing [souretsu] model. Table A6. 1Wanting one complete mixing tank and be making (The reluctance tank: one division and hypoxia tank: four divisions and conceited tanks: four divisions) severally of the shown [ni] each division. ・ The final sedimentation tank was modeled as an ideal sedimentation pond where the floatage element did not flow out to the treated water. However, it tempered with the phenomenon in the waste treatment facility of the reality, and DO(SO2 ) of the sending back dirt and nitrate-nitrogen (SNO3 ) were compulsorily 0 in facilities A and B It fixed to mg/L. The purpose of this is to exclude a part of an indirect influence through the sending back dirt water quality when parameter value etc. are changed. ・ The time varying was not given to all the flow rate (influent, sending back dirt, 413 Appendix 6Sensitivity analysis example dirt pulling out, and internal circulation), and it was assumed the definite value. ・ SRT (And, ASRT) : the dirt pulling out flow rate. Table A6. 1It was set that it became a shown [ni] numerical value. A numerical value different in facilities A・B where nitrification was required and facilities C・D not so was set to ASRT (14d and 5 eachd). Because HRT of a conceited tank is assumed to be identical in all facilities, the difference of SRT by the difference of the solid concentration of a reactive tank will be chiefly expressed. ・ The water temperature was fixed to 20℃. ASRT set in facilities A・B is making the maintenance of nitrifying bacteria at design water temperature (13 ℃ ), assumption it can be said the condition with room as capacity of a reactive tank. ・ The DO(SO2 ) density of a conceited tankTable A6. 1It fixed compulsorily to the shown [ni] numerical value. Because the DO density was required to reflect the realities in the calibration as well as the sending back dirt water quality, an indirect influence through this was excluded. This DO set point was a condition of evenly supplying air to each division of a conceited tank in facilities A, and DO in the conceited tank end was 1.5 It set it from the DO density of a conceited each tank when the state to control a total blast volume to become mg/L was simulated (All the default value was used for the parameter), and the calculation result reached regularly. Table A6. 1Outline of virtual facilities made object of sensitivity analysis Facilities name Facilities A Facilities B Facilities C Facilities D Processing mode Conceited reluctance-hypoxia-m ethod Circle method nitrification denitrification method Conceited reluctance-active sludge process Conventional activated sludge process Reactive tank Composition Reluctance ×1 Hypoxia × four conceit ×4 Hypoxia ×4 Conceit ×4 Reluctance ×1 Conceit ×4 Conceit ×4 Reactive tank HRT 15.5h (8h of reluctance 1.5h+ hypoxia 6h+ conceit) 14h (8h of hypoxia 6h+ conceit) 11.3h (8h of reluctance 3.3h+ conceit) 8h (conceit 8h) SRT(ASRT) 27d(14d) 24.5d(14d) 7.1d(5d) 5d(5d) Dirt sending back ratio Internal circulation ratio 0.5 1.5 - 414 Appendix 6Sensitivity analysis example Water temperature 20℃ Conceited tank DO*1 The first division: 0.3 mg/L and the 2nd division: 0.5 mg/L and the 3rd division: 0.7 mg/L and the 4th division: 1.5 Mg/L Influent two ..quality *.. [mg/L] T-COD:235, SI :23, SF :2.4, SA :9.6, XI :35, XS :135, XH :30 XAUT・XPAO :0.2, SNH4 :22.6, SNO3 :0, SPO4 :1.9, SALK :250 Reactive tank end water quality mg/L in basic condition SNH4 :1.2, SNO3 :6.6,SPO4 :0.8 , XI :2,370,XS :24, XH :680, XPAO :330, XPP :99XPHA :0.3, XAUT :55, XTSS :3,080 SNH4 :0.8, SNO3 :6.7,SPO4 :1.8 , XI :2,440,XS :25, XH :850, XPAO :190, XPP :58XPHA :0.2, XAUT :61, XTSS :3,030 SNH4 :2.4, SNO3 :11.3SPO4 :0. 8, XI :750,XS :18, XH :753, XPAO :89, XPP :27XPHA :0.1, XAUT :43, XTSS :1,460 SNH4 :0.9, SNO3:16.0,SPO4 :2. 3, XI :724,XS :22, XH :980, XPAO :3, XPP :0.8XPHA :0.0, XAUT :52, XTSS :1,490 - 1 Compulsion fixed value. - 2 Basic condition. It sets it on the basis of a sedimentation pond overflow average at the beginning composition in investigation of actual conditions. Appendix 6Sensitivity analysis example 415 ・ First of all, when the organism element density of influent is set, do investigation of actual conditions result (..BOD5 used by the calculation of capacitance.. "by JS. Error! Reference source not found. Error! Reference source not found.It converted into CODCr on the basis of Refer to"), and each element density was set on the basis of an average water quality composition of [shizugomizu] that had been obtained in addition for investigation of actual conditions. On the other hand, what used by the calculation of capacitance was used as it was for nitrogen and the Rin element density. The time varying was not given to the influent quality as well as the influent quantity. Object and evaluation method of sensitivity analysis Table A6. 1In shown inflow condition and [ni] operating condition, the condition using the default value was assumed to be "Basic condition" to all parameters. On the other hand, the element density when the simulation that individually changed the following analysis objects respectively by ±20% was executed, and the calculation reached regularly was used for the evaluation. ‒ All parameters of ASM2d (kinetics constant, amount of theory coefficient, and conversion factor) ‒ Organism element density of influent (SA ,SF ,XI ,XS ,XH ) ‒ Operating condition etc. (water temperature, influent quantity, dirt sending back ratio, internal circulation ratio, dirt pulling out flow rate, and conceited tank DO density) The element used as an evaluation target is SNH4, SNO3, SPO4, and XTSS of the reactive tank each division. The following indices were used to evaluate that the simulation result was the impact combination about each analysis object (sensitivity). ・ Amount (AbSp,n,y ) of the average relative change: Expression in the mean though the variation in reactive tank division n to the basic condition of element y was divided in the variable ratio of a function of change of analysis object p(+20% or -20% in case of this discussion). Expression (A6. 1)Each analysis object and each element of the [niyori] each reaction tank for the evaluation were calculated. A negative value is taken in the axis of abscissas when positive and decreasing 416 Appendix 6Sensitivity analysis example when y increases when it corresponds to the inclination of the straight line when element y is plotted in the variable ratio of a function of change and the spindle of analysis object p, and p is increased. It uses it as a basic index to express the sensitivity of each analysis object in this discussion. pdef AbS p ,n, y Ave y p Expression (A6. 1) 417 Appendix 6Sensitivity analysis example It is ?y here: the variation and pdef to the basic condition of element y: It is variation of p to the value of analysis object p in the basic condition and ?p:pdef. ・ Sensitivity uniqueness (SPy,p ): In the reactive each tank division, it is an expression as the index that expresses analysis object p influences element y in the peculiarity very (Compared with the influence on other elements for the evaluation). Expression (A6. 2)[Niyori] was calculated. It means analysis object p influences only element y in the peculiarity by taking the range of 0-1, and the numerical value large (It is near one) as for this index in the division. ReS ReS 2 SPp ,n , y p ,n, y 2 Expression (A6. 2) p ,n ,k k ReSp, n, and y here: ..(of the rate of the average relative change.. expression of y in division n by p. Expression (A6. 3))The denominator will calculate the ratio of contribution of the y p def AbS p ,n , y Re S p ,n , y Ave y def y def p Expression (A6. 3) It is ydef here: It is element y density in the basic condition. Calculation way The calculation condition in the simulation is as follows. Note the point evaluated when it is not the process but it calculates on the 90th and each element density reaches roughly regularly though each element density begins to change according to it when the analysis object is changed from the basic condition. ‒ Numerical calculation: Runge-the fourth Kutta methods ‒ Width (*t) of time interval in numerical calculation: 10s ‒ Initial condition: Stationary solution in basic condition ‒ Simulation period: 90d 418 Appendix 6Sensitivity analysis example (3)Result of review Simulation result in basic condition Because it evaluates that it is the impact combination about the analysis object by the variation and the variable ratio of a function of change to the basic condition in the sensitivity analysis, it is important to consider the simulation result in the basic condition enough. The water quality profile of a reactive tank of facilities A-D in the basic conditionFigure A6. 1Each element density of [ni] and the reactive tank end divisionTable A6. 1[Ni] was shown. The latter half of this tank becomes an anoxic because there is room in the capacity of the hypoxia tank, and the feature the point that the discharge of then remarkable SPO4 is caused though a water quality changing typical a conceited reluctance-hypoxia-method like nitrification, the denitrification, the discharge of PO4-P(SPO4 ), and the incorporation, etc. is seen in facilities A. The hypoxia tank latter part of facilities B where the biological phosphorus removal is not considered on the facilities parameter substantially functions similarly to this as a reluctance tank, and the calculation result (The proportion of XPAO in XTSS equals facilities C of a conceited reluctance-method) to which some biological phosphorus removal progresses. In facilities C, the biological phosphorus removal is excellently caused, and nitrification progresses, too. However, the SNH4 density of the treated water is a little high compared with other facilities. It is a calculation result that XPAO doesn't exist in the faction because there is no division that becomes an anoxic also in facilities D though nitrification progresses excellently. Sensitivity to nitrification The NH4-N(SNH4 ) density of the reactive tank end division of the influence on the nitrification of parameter value etc. that become analysis objects is arranged as the main index. 419 Appendix 6Sensitivity analysis example Table A6. 2Sensitivity (The amount of the average relative change; AbSp, n, and y) and sensitivity uniqueness (SPp,n,y ) were shown about SNH4 of the reactive tank end division of each facilities of ..peel.. ,. The parameter of ASM2d is extracted only the one of 0.5 or more by sensitivity, divided on the basis of the involved reaction process, and displayed. On the other hand, the result of all analysis objects is published about the influent quality and the operating condition. ・ Sensitivity with a big parameter concerning XAUT is shown and the sensitivity uniqueness is also high (It influences SNH4 in the peculiarity) in each facilities. Especially, sensitivity that μAUT that changes the proliferation rate of XAUT (= nitrification rate) directly is the maximum in any facilities is shown. Moreover, the sensitivity of bAUT is common, high, too and it is shown that the amount of XAUT in the faction greatly influences SNH4 of the treated water. The sensitivity of various saturation factor that defines that excluding this, the proliferation rate of XAUT such as KO2, KNH4, and KALK is the impact combination about the environmental factor is comparatively high. ・ The sensitivity of the above-mentioned parameter is growing in facilities where the density of treated water SNH4 in the basic condition is high, and the calculation result according to nitrification is overall sensitive to parameter value. In this, in the simulation usage in which the marginal condition to maintain nitrifying bacteria in the faction is found, it is suggested that the prediction result greatly depend on parameter value, and attention is a necessary point. 25 25 SNH4 20 SNH4 20 SNO3 [mg/L] 15 SNO3 [mg/L] 15 SPO4 10 10 5 5 0 SPO4 0 1 2 3 4 5 6 7 Reactive tank division (a) Facilities A 8 9 1 2 3 4 5 6 Reactive tank division (b) Facilities B 7 8 420 Appendix 6Sensitivity analysis example 25 SNH4 SNO3 25 20 SPO4 SNH4 20 SNO3 [mg/L] 15 [mg/L] 15 SPO4 10 10 5 5 0 1 0 1 2 3 4 5 2 3 4 Reactive tank division Reactive tank division (c) Facilities C (d) Facilities D Figure A6. 1Simulation result by basic condition (reactive tank water quality profile) ・ There is something that shows SNH4 big sensitivity also in the parameter concerning XPAO in facilities A and C. This is because the limitation paragraph according to SPO4 greatly influences in the rate equation in the proliferation process of XAUT the SPO4 density of the conceited tank latter part is small in both facilities. Because the nitrification rate doesn't decrease remarkably even if SPO4 becomes a low concentration, such a phenomenon is actually prevented being caused by reducing KP (KP,A ) in this process in the calibration. ・ The influence of the influent organism element density is a small overall. ・ The DO ..pulling out.. density of dirt of [haka] and a conceited tank comparatively shows big sensitivity in the operating condition etc.As for the former, if it is considered that ..dirt.. ..pulling out.. [haka] in the final sedimentation pond model used in this discussion decides SRT, it is a result of it is easy to understand. If the DO density of the object facilities cannot be understood on such a condition, the uncertainty at the calibration extremely grows though the influence of the DO density is growing by equaling to parameter value. Sensitivity to denitrification Sensitivity to the SNO3 density of the reactive tank end division and the sensitivity uniqueness as an influence on the denitrification as well as the preceding clause Appendix 6Sensitivity analysis example 421 Table A6. 3[Ni] was brought together. ・ Generation by nitrification contributes chiefly, and many of parameters concerning XAUT show big sensitivity here for the change in a conceited tank in the SNO3 density. Validity that does the calibration of nitrification before the denitrification is here. ・ In other parameters, the parameter that influences the denitrification rate such as ηNO3 and KO2 concerning XH directly comparatively shows big sensitivity, and the sensitivity uniqueness is also high. ・ This is because the density of the total nitrogen that flows in chiefly changes though conversion factor iN and Xs comparatively show big sensitivity. As for the point where the sensitivity of the XS density of influent is large, it is similar. ・ In the operating condition etc. , the internal circulation ratio in facilities A・B in addition to [haka] and the DO density shows big sensitivity in the preceding clause pulling out ..showing.. ..dirt... This is because the SNO3 load to the hypoxia tank is chiefly decided depending on the internal circulation ratio, and a common characteristic to the process of a hypoxia tank prepositive type. Sensitivity to phosphorus removal Sensitivity to the SPO4 density of the reactive tank end division and the sensitivity uniqueness as an influence on the biological phosphorus removal 422 Appendix 6Sensitivity analysis example Table A6. 4[Ni] was shown. About the influence of the parameter concerning XPAO, "Error! Reference source not found.Error! Reference source not found.Refer to". ・ Sensitivity with a big a lot of parameters is shown excluding facilities D where XPAO doesn't exist in the faction. ・ It has sensitivity with a big parameter related to hydrolysis involved in the substrate supply to XPAO in addition to the parameter that changes the behavior of XPAO, and the sensitivity uniqueness is also high. Appendix 6Sensitivity analysis example 423 ・ There is something that shows sensitivity big as for the parameter concerning XH. As for XH, only it is not in XPAO and the competition over the organic substrate, and because the substrate supply to XPAO as the subject of hydrolysis and the fermentation process is borne, the way of the influence appearance is complex. ・ Conversion factor iP and Xs see to change the amount of total phosphorus that flows in as well as nitrogen and have big the above sensitivity. ・ SA and XS comparatively show big sensitivity, and it is suggested that the behavior of XPAO be limited by supplying the organic substrate in each facilities in the influent organism element. As for the influences of the organism element density except XI, the majority appear to the phosphorus removal (The sensitivity uniqueness to SPO4 is high). ・ The influence of the operating condition etc. is also larger than that of nitrification and the denitrification. Sensitivity to abundance of solid Sensitivity to the XTSS density of the reactive tank end division and the sensitivity uniqueness as an influence on the abundance of the solid 424 Appendix 6Sensitivity analysis example Table A6. 5[Ni] was shown. Because SRT is fixed in the simulation condition used by this discussion, the XTSS density of a reactive tank can be handled as an index that reflects the abundance of the solid directly. ・ It has sensitivity with big growth yield and ratio autolysis speed that influences the amount in the faction of the main living being element (XH and XPAO) in the parameter. ・ Because the amount of autolysis relatively grows in facilities A・B where SRT is long, the sensitivity of fXI that defines the XI yield according to autolysis is growing. ・ The sensitivity of XI and XS is large in the influent organism element. XI is a parameter that influences only the abundance of the solid in the peculiarity. On the other hand, XS influences the biomass in the faction and the amount of XI of the autolysis origin as the main organism element of influent. ・ The sensitivity of [haka] ..the influent quantity, the dirt sending back ratio, and dirt.. ..pulling out.. is large in the operating condition. The influence of the inflow load remarkably appears to the solid concentration of a reactive tank because SRT is kept constant though the inflow load volume changes by changing the influent quantity in this discussion condition. On the other hand, ..pulling out ..the dirt sending back ratio and dirt.... [haka] influences the abundance of the solid because it changes SRT. Sensitivity to treated water SNH4 and the SPO4 density when three of the conditions setting the influent organism element density by using the component ratio illustrated in the condition of reducing condition (ASRT=14d→9d) with different (a) SRT and the capacity of the (b) hypoxia tank to 60% and the (c) technical report about facilities A referring executes a similar sensitivity analysis respectively as a basic condition Appendix 6Sensitivity analysis example Table A6. 6、 425 426 Appendix 6Sensitivity analysis example Table A6. 7[Ni] was published. If the facilities condition, the inflow condition, and the operating condition, etc. are different even if it is the same processing mode, the point where the sensitivity analysis result is different should be able to be understood. 427 Appendix 6Sensitivity analysis example Table A6. 2Summary of sensitivity to SNH4 in reactive tank end Analysis object ....drinkin g.. [ku].. Addi tion of wate r Res oluti on Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on Facilities B (circle method nitrification denitrification method) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on Facilities C (conceited reluctance-active sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati Kh on 1.12 2% ηfe 1.10 2% Facilities D (conventional activated sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on Parameter XH Rela tion XPAO Rela tion YPAO 0.75 2% μAUT bAUT KO2 KNH4 KALK -15.07 7.18 3.24 1.81 0.96 99% 99% 99% 99% 99% μAUT bAUT KO2 KNH4 KALK -7.02 3.66 1.82 1.22 0.55 100% 100% 100% 100% 100% Inflo w Wat er quali ty SF SA XI XS XH 0.00 -0.02 0.00 0.06 0.00 0% 0% 0% 0% 0% SF SA XI XS XH 0.00 -0.02 0.00 -0.04 0.00 0% 0% 0% 0% 0% -0.11 27% -0.06 76% -0.04 0% 0.01 0% Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -0.93 21% -0.50 57% 0.17 1% 0.18 3% 1.52 24% 0.86 60% -3.76 91% -2.05 94% XAUT Rela tion YPAO bPAO qPHA 41.12 -1.06 0.68 87% 2% 0% μAUT bAUT KO2 KNH4 KALK YA -54.39 16.50 11.11 3.35 2.64 0.76 97% 88% 86% 83% 83% 43% μAUT KO2 bAUT KNH4 KALK -10.69 2.35 2.33 1.28 0.74 100% 100% 100% 100% 100% 0.04 0.08 0.00 1.63 0.08 0% 0% 0% 3% 0% SF SA XI XS XH 0.00 -0.04 0.00 -0.05 0.01 1% 36% 0% 1% 1% -0.86 74% -0.25 99% 0.08 0% 0.10 2% -12.47 66% -2.03 95% - - - - 11.85 91% 3.15 95% -25.23 89% -2.61 97% Oth ers Other input data Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO SF SA XI XS XH Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Basic condition 1.21 mg/L 0.77 mg/L 2.39 mg/L 0.85 mg/L [Deno] - density The amount of the average relative change displays it, and the small one and the order display from 0.5 and the 16th place following are not displaying the parameter. 428 Appendix 6Sensitivity analysis example Table A6. 3Summary of sensitivity to SNO3 in reactive tank end Analysis object ....drinkin g.. [ku].. Addi tion of wate r Res oluti on Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on Facilities B (circle method nitrification denitrification method) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on ηNO3 KO2 Parameter KO2 ηNO3 -1.26 -1.25 18% 88% ηNO3 -0.51 3% μAUT bAUT KO2 KNH4 4.26 -1.87 -0.68 -0.54 0% 0% 0% 0% μAUT bAUT 1.70 -0.74 iN,Xs 1.76 32% iN,Xs Inflo w Wat er quali ty SF SA XI XS XH -0.02 -0.17 0.00 -0.77 0.16 0% 0% 0% 0% 0% SF SA XI XS XH 0.03 0% 0.05 0% Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -0.80 1% -2.91 11% -0.50 0% 2.55 1% XH Rela tion XPAO Rela tion XAUT Rela tion Oth ers Other input data Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -1.50 -1.49 81% 25% Facilities C (conceited reluctance-active sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati Kh -0.81 on 0% ηfe -0.62 0% ηNO3 KO2 YH bH -5.74 -5.73 2.33 0.63 91% 91% 1% 1% 1% 1% 1% 1% 1% 2% μAUT KO2 bAUT KNH4 KALK YA 9.92 -2.13 -2.13 -1.21 -0.68 -0.53 0% 0% 0% 0% 0% 2% 3.45 -1.32 8% 8% iN,Xs iN,BM fXI 5.07 -2.60 0.60 96% 96% 31% -0.07 -0.42 0.00 -2.93 0.14 0% 0% 0% 0% 0% SF SA XI XS XH -0.13 -0.74 0.00 -4.30 0.05 43% 33% 0% 22% 43% 0.52 1% 0.23 0% -0.01 0% 0.43 0% 4.87 0% 1.95 0% - - - - -7.26 2% -2.98 0% 17.38 2% 8.11 3% KO2 ηNO3 -2.67 -2.62 5% 29% YPAO bPAO -23.76 0.67 1% 0% 0% 0% μAUT bAUT KO2 KNH4 KALK YA 28.65 -9.73 -6.29 -2.07 -1.57 -0.67 1.76 36% iN,Xs iN,BM -0.02 -0.16 0.00 -0.78 0.14 0% 1% 0% 1% 0% SF SA XI XS XH 0.02 0% 0.02 0% -0.95 3% -2.97 12% -0.28 0% 2.02 1% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Facilities D (conventional activated sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati Kh -1.07 on 89% KX 0.58 88% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Basic condition 6.59 mg/L 6.65 mg/L 11.29 mg/L 16.01 mg/L [Deno] - density The amount of the average relative change displays it, and the small one and the order display from 0.5 and the 16th place following are not displaying the parameter. 429 Appendix 6Sensitivity analysis example Table A6. 4Summary of sensitivity to SPO4 in reactive tank end Analysis object ....drinkin g.. [ku].. Addi tion of wate r Res oluti on XH Rela tion Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe -1.63 on 100% Kh -1.42 100% KX 0.59 100% Facilities B (circle method nitrification denitrification method) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati Kh -1.66 on 99% ηfe -1.63 99% ηNO3 -0.89 99% KX 0.83 99% Parameter Other input data YH bH -2.16 0.61 93% 96% μH KO2 0.67 -0.67 99% 73% YPAO KMAX bPAO qPP YPO4 μPAO qPHA -3.58 -1.93 1.81 -1.37 1.07 0.95 -0.59 97% 100% 98% 100% 99% 100% 100% YPAO bPAO qPHA KMAX qPP KA YPO4 -3.14 1.90 -1.39 -1.24 -0.78 0.77 0.71 98% 99% 99% 99% 99% 99% 99% XAUT Rela tion μAUT 0.75 1% Oth ers iP,Xs 1.27 99% iP,Xsi N,Xs 1.33 0.63 Inflo w Wat er quali ty SF SA XI XS XH -0.08 -0.44 0.00 -2.00 -0.08 99% 99% 0% 96% 99% SF SA XI XS XH -0.10 -0.56 0.00 -2.59 -0.02 Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO XPAO Rela tion 0.12 72% 0.62 40% 1.13 70% 1.03 88% -1.75 69% 0.72 7% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Facilities C (conceited reluctance-active sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati Kh -2.79 on 98% ηfe -2.78 98% KX 1.21 99% Facilities D (conventional activated sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on YH bH -1.95 0.53 44% 51% YPAO qPHA bPAO μPAO qPP KA YPO4 KMAX -4.89 -3.48 2.25 0.94 -1.20 1.16 1.13 -1.47 12% 100% 98% 100% 99% 99% 99% 99% μAUT bAUT KO2 2.43 -1.83 -1.40 2% 11% 13% 99% 64% iP,Xs 1.28 99% iP,Xs iP,BM 1.33 -0.77 99% 100% 95% 96% 0% 88% 95% SF SA XI XS XH -0.18 -0.87 0.00 -3.19 -0.27 99% 100% 0% 95% 99% SF SA XI XS XH 0.01 -0.07 0.00 0.35 0.25 5% 14% 0% 7% 5% 0.03 0% -0.03 0% 0.36 0% 0.08 22% 0.84 21% 0.39 7% 2.04 84% -0.94 13% 1.10 5% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO 0.16 24% 0.92 63% 2.84 33% - - -0.90 5% 2.56 9% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO - - -0.56 0% -0.01 0% Basic condition 0.81 mg/L 1.76 mg/L 0.76 mg/L 2.30 mg/L [Deno] - density The amount of the average relative change displays it, and the small one and the order display from 0.5 and the 16th place following are not displaying the parameter. 430 Appendix 6Sensitivity analysis example Table A6. 5Summary of sensitivity to XTSS in reactive tank end Analysis object ....drinkin g.. [ku].. Parameter Addi tion of wate r Res oluti on XH Rela tion Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe on 235 0% Facilities B (circle method nitrification denitrification method) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe on 235 1% Kh 215 1% Facilities C (conceited reluctance-active sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on YH bH 2,230 -462 7% 4% YH bH 2,495 -525 95% 36% YH bH YPAO bPAO KMAX 1,128 -414 243 1% 0% 0% YPAO bPAO qPHA 773 -358 205 2% 1% 1% Oth ers iTSS,XI iTSS,BM fXI 1,809 960 436 100% 100% 15% iTSS,XI iTSS,BM fXI 1,826 996 435 Inflo w Wat er quali ty SF SA XI XS XH 33 134 1,062 1,492 449 1% 1% 100% 4% 1% SF SA XI XS XH 36 154 1,075 1,620 457 Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO XPAO Rela tion 1,180 -310 73% 4% YPAO 366 0% 100% 100% 33% iTSS,BM iTSS,XI 796 564 100% 100% 5% 2% 100% 12% 5% SF SA XI XS XH 21 86 393 835 254 0% 0% 100% 2% 0% Facilities D (conventional activated sludge process) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on YH bH 1,401 -327 54% 46% iTSS,BM iTSS,XI 928 543 100% 100% SF SA XI XS XH 13 50 393 722 254 50% 17% 100% 71% 50% XAUT Rela tion Other input data -50 1% 2,894 60% 1,454 8% -154 0% -2,216 8% -97 0% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -45 3% 2,808 79% 1,509 33% -301 1% -2,265 27% -158 0% Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -25 0% 1,362 37% 593 0% - - -1,110 2% -95 0% Basic condition 3,080 mg/L 3,030 mg/L 1,460 mg/L [Deno] - density The amount of the average relative change doesn't display the small one of the parameter from 200. Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -20 0% 1,444 98% 722 4% - - -1,131 4% 4 0% 1,490 mg/L 431 Appendix 6Sensitivity analysis example Table A6. 6Comparison of sensitivity to SNH4 in reactive tank end when condition of facilities A is changed Analysis object ....drinkin g.. [ku].. Addi tion of wate r Res oluti on Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on XH Rela tion Parameter Other input data YH 10.19 13% YH 1.83 7% YPAO μPAO qPP YPO4 KMAX bPAO 6.89 -2.35 2.24 -1.87 1.42 -1.40 12% 7% 6% 12% 3% 7% μAUT bAUT KO2 KNH4 KALK -25.08 10.09 4.17 2.13 1.15 99% 98% 98% 6% 98% iP,Xs -0.96 9% SF SA XI XS XH 0.58 0.47 0.00 1.03 0.01 7% 5% 0% 4% 7% -0.25 27% -0.46 10% -2.54 29% -0.43 4% 3.68 17% -5.51 81% 7% 7% YPAO μPAO qPP YPO4 KMAX bPAO 44.10 -36.22 27.29 -13.34 4.85 -4.53 36% 67% 44% 34% 4% 6% μAUT bAUT KO2 KNH4 KALK -15.07 7.18 3.24 1.81 0.96 99% 99% 99% 99% 99% μAUT bAUT KO2 KNH4 KALK -53.51 38.95 17.91 4.80 3.80 95% 92% 86% 75% 76% iP,Xs -4.26 13% SF SA XI XS XH 0.32 1.45 0.00 8.77 0.66 11% 9% 0% 7% 11%% SF SA XI XS XH Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO Basic condition [Deno] Reactor End water quality ηfe Kh 2% Inflo w Wat er quali ty 0.00 -0.02 0.00 0.06 0.00 0% 0% 0% 0% 0% -0.11 27% -0.04 0% -0.93 21% 0.17 1% 1.52 24% -3.76 91% SNH4 :1.2, SNO3 :6.6,SPO4 :0.8, XI :2,370,XS :24, XH :680, XPAO :330, XPP :99XPHA :0.3, XAUT :55, XTSS :3,080 Condition A-4 (technical report segmentation ratio)- 2 Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe on 1.07 5% Kh 0.85 6% Sensiti vity Uniqu eness 0.75 Oth ers Condition A-3 (capacity of hypoxia ×60%)- 1 Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati on Avera ge Relativ ity Variati on 6.12 5.31 Analys is Object YPAO XPAO Rela tion XAUT Rela tion Condition A-2 (ASRT:14d→9d) Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -0.98 36% -2.00 15% -13.02 56% -3.59 9% 19.56 44% -29.99 83% SNH4 :3.1, SNO3 :6.0,SPO4 :0.1, XI :1,480,XS :23, XH :610, XPAO :290, XPP :77,XPHA :0.4, XAUT :45, XTSS :2,230 μAUT bAUT KO2 KNH4 SF SA XI XS XH Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -4.80 2.59 1.34 0.98 0.00 -0.02 0.00 -0.02 0.00 100% 100% 100% 100% 0% 1% 0% 0% 0% -0.05 62% 0.01 0% -0.39 48% 0.15 5% 0.68 53% -1.50 94% SNH4 :0.6, SNO3 :6.7,SPO4 :1.5, XI :2,420,XS :25, XH :880, XPAO :230, XPP :69,XPHA :0.1, XAUT :64, XTSS :3,110 Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO SNH4 :1.4, SNO3 :6.3,SPO4 :0.2, XI :1,910,XS :24, XH :620, XPAO :450, XPP :122,XPHA :0.5, XAUT :54, XTSS :2,860 432 Appendix 6Sensitivity analysis example - The amount of the average relative change displays it, and the small one and the order display from 0.5 and the 16th place following are not displaying the parameter. - 1 The capacity of the hypoxia tank of four divisions is reduced to 60% respectively. - 2 The influent quality is set on the basis of the organism element composition ratio illustrated in the technical report of ASM2 (SF :27 mg/L, SA :18 mg/L, SI :27 mg/L, XI :23 mg/L, XS :113 mg/L, and XH :27 mg/L; Total CODCr is 235 It is the same as other conditions in mg/L). 433 Appendix 6Sensitivity analysis example Table A6. 7Comparison of sensitivity to SPO4 in reactive tank end when condition of facilities A is changed Analysis object ....drinkin g.. [ku].. Addi tion of wate r Res oluti on XH Rela tion Condition A-2 (ASRT:14d→9d) Sensiti vity Uniqu eness ηfe Kh Avera ge Relativ ity Variati -0.95 on -0.83 Analys is Object 93% 93% Condition A-3 (capacity of hypoxia ×60%)- 1 Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe -1.70 on 100% Kh -1.69 100% KX 0.78 100% ηNO3 -0.64 100% Condition A-4 (technical report segmentation ratio)- 2 Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe -0.59 on 95% Parameter Other input data YH bH -2.16 0.61 93% 96% YH -1.12 86% YH μH bH -1.45 0.62 0.56 58% 100% 82% YH -0.89 92% YPAO KMAX bPAO qPP YPO4 μPAO qPHA -3.58 -1.93 1.81 -1.37 1.07 0.95 -0.59 97% 100% 98% 100% 99% 100% 100% YPAO qPP μPAO KMAX YPO4 bPAO -2.53 -1.32 1.07 -1.06 0.81 0.78 63% 55% 31% 96% 66% 94% YPAO bPAO KMAX qPHA qPP YPO4 KA -3.26 2.13 -1.46 -1.29 -0.97 0.82 0.65 98% 99% 100% 100% 100% 99% 100% YPAO qPP μPAO KMAX YPO4 bPAO -2.49 -1.17 1.14 -1.07 0.68 0.66 88% 94% 93% 97% 88% 93% XAUT Rela tion μAUT 0.75 1% Oth ers iP,Xs 1.27 99% iP,Xs 1.32 99% Inflo w Wat er quali ty SF SA XI XS XH -0.08 -0.44 0.00 -2.00 -0.08 99% 99% 0% 96% 99% SF SA XI XS XH -0.10 -0.53 0.00 -2.17 -0.01 97% 97% 0% 89% 97% SF SA XI XS XH -0.29 -0.27 0.00 -0.63 -0.02 93% 94% 0% 94% 93% 0.05 73% -0.03 2% Drivi ng Con ditio n [Na], [do] Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO 0.52 69% 0.26 88% -1.07 82% 0.34 18% XPAO Rela tion Basic condition [Deno] Reactor End water quality - Facilities A (conceited reluctance-hypoxia-metho d) Avera Sensiti Analys ge vity is Relativ Uniqu Object ity eness Variati ηfe -1.63 on 100% Kh -1.42 100% KX 0.59 100% 0.12 72% 0.62 40% 1.13 70% 1.03 88% -1.75 69% 0.72 7% SNH4 :1.2, SNO3 :6.6,SPO4 :0.8, XI :2,370,XS :24, XH :680, XPAO :330, XPP :99XPHA :0.3, XAUT :55, XTSS :3,080 SF SA XI XS XH Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO -0.04 -0.20 0.00 -1.43 -0.08 88% 90% 0% 93% 88% 0.06 63% 0.16 51% 0.49 42% 0.50 91% -0.94 54% 0.55 15% SNH4 :3.1, SNO3 :6.0,SPO4 :0.1, XI :1,480,XS :23, XH :610, XPAO :290, XPP :77,XPHA :0.4, XAUT :45, XTSS :2,230 Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO 0.09 36% 0.80 25% 0.65 22% 0.65 79% -1.07 23% 0.83 5% SNH4 :0.6, SNO3 :6.7,SPO4 :1.5, XI :2,420,XS :25, XH :880, XPAO :230, XPP :69,XPHA :0.1, XAUT :64, XTSS :3,110 Water tempe rature Inlet flow Sendi ng back ratio Circul ation ratio ..pullin g out.. [haka] DO SNH4 :1.4, SNO3 :6.3,SPO4 :0.2, XI :1,910,XS :24, XH :620, XPAO :450, XPP :122,XPHA :0.5, XAUT :54, XTSS :2,860 The amount of the average relative change displays it, and the small one and the order display from 0.5 and the 16th place 434 Appendix 6Sensitivity analysis example following are not displaying the parameter. - 1 The capacity of the hypoxia tank of four divisions is reduced to 60% respectively. - 2 The influent quality is set on the basis of the organism element composition ratio illustrated in the technical report of ASM2 (SF :27 mg/L, SA :18 mg/L, SI :27 mg/L, XI :23 mg/L, XS :113 mg/L, and XH :27 mg/L; Total CODCr is 235 It is the same as other conditions in mg/L). 435 Appendix 6Sensitivity analysis example 3.Detailed sensitivity analysis on parameter according to biological phosphorus removal (1)Outline A lot of processes and parameters are used for the biological phosphorus removal model of ASM2d. It is easy to be going to spend the labor that number of degrees of freedom at the calibration is high and large because a lot of parameters have a big influence from the sensitivity analysis result of propodus on the simulation result like clearness. There is the interaction between complex processes, and the behavior of the simulation result when parameter value is changed is and is intuitively a part not understood easily either there is a competition over organic substrate (XPHA ) like "Store of XPP" and process that XPAO bears "Proliferation of XPAO", etc. The parameter involved in the simulation result of the biological phosphorus removal from the result of review of propodus is extracted, and it introduces the result of discussing the influence more in detail from such circumstances here. (2)Review method Object facilities and process model (..using facilities parameter and process model of facilities A (conceited reluctance-hypoxia-method) used by discussing propodus..Table A6. 1)。 Object parameter and width of change The object was selected centering on the parameter that comparatively showed big sensitivity to the simulation result related to the phosphorus removal by discussing propodus. However, the possibility of changing it by the process of the calibration such as YPAO excluded the small one and the conversion factor. The simulation was executed within each parameter value-..individual.. range of 80%-+100% at intervals of 10% on the condition of changing it, and each stationary solution (element density after it had simulated it ..between 90d..) was used for the analysis and the evaluation. The calculation way in the simulation is identical with the discussion about propodus. (3)Result of review The appearance of the change in SPO4 in the SPO4 density of the SPO4 density of the (a) reaction tank each division when each parameter value is changed (PO4-P profile) 436 Appendix 6Sensitivity analysis example and the (b) reluctance tank and a conceited tank ends, XPAO, and the XH densityFigure A6. 2~Figure A6. 14[Ni] was shown. Appendix 6Sensitivity analysis example 437 Behavior of anoxic ・ The parameter concerning the store process of the parameter concerning hydrolysis involved in the substrate supply to XPAO there (Kh, KX, and ηfe, etc.) and XPAO (qPHA and YPO4, etc.) greatly influences Rin's discharge under the anoxic. ・ It comparatively resembles it, and the phosphorus removal is shown and the method of the influence shows the promotion or the effect of depraving it overall though parameters except YPO4 are different among these the size of each influence it. For instance, (..increasing SPO4 burst size under the anoxic when it is changed into the direction in which it promotes it that these phosphorus removals (Kh, ηfe, increase of qPHA, and decrease of KX).. ..the SPO4 incorporation under aerobic condition hypoxia/.. ..the promotion.. ..the final reduction of the SPO4 density of the treated water... Figure A6. 2~Figure A6. 5)。 This increases the amount of the organism for which XPAO can be used, and is corresponding (Here, the competition between XPAO and XH over the organic substrate is important) the growth yield of XPAO (XPAO density) is increased. ・ SA and the density of SF of influent grow in the condition of this discussion and the contribution of the hydrolysis process is growing relatively compared with the substrate supply in the small straightening and the anoxic. Moreover, it is hydrolyzed before it arrives at a conceited tank, and the increase and decrease of the hydrolysis rate by the anoxic by the increase and decrease of the speed of the entire hydrolysis process by Kh and ηfe is the results of it having a roughly equal influence in the majority of XS of the influent origin. ・ ..density of SF of influent.. (of ..parameter's concerning the small straightening and the fermentation process (qfe etc.) influence.. small in the condition of this discussion. Figure A6. 6)。 ・ YPO4 changes the amount of the phosphorus release for each organic substrate uptake by XPAO, and doesn't influence direct in the uptake of the organic substrate. Therefore, it influences SPO4 burst size under the anoxic in the peculiarity comparatively. (..the influence on the XPAO density and the amount of the endogenous substrate.. ..moving of the SPO4 profile of a small straightening and a reactive tank comparatively overall up and down... Figure A6. 13)。 ・ Because the above-mentioned parameter influences the entire SPO4 profile of a reactive tank, the good policy in the calibration doing the fitting of the anoxic before hypoxia/aerobic condition. 438 Appendix 6Sensitivity analysis example Behavior of hypoxia/aerobic condition ・ As the behavior of XPAO, under aerobic condition hypoxia/the store and the proliferation process of XPP are subjects. The parameter involved in these processes doesn't influence the behavior of SPO4 under the anoxic too much. ・ Both processes are in the relation that competes over intracellular store organism XPHA of XPAO. When the speed of either of process is changed because XPHA often becomes the limiting factor of both processes in the vicinity of the reactive tank end, the other process is influenced, too. In addition, an interaction complex as the amount of XPAO changing when the speed of the proliferation process changes influences the speed of both processes is caused. However, it is easy to understand it usually pays attention to SPO4 because the variable ratio of a function of change of SPO4 is larger than the variable ratio of a function of change of XPAO and the calibration is done (The case where the amount of autolysis of XPAO is changed by bPAO is excluded). ・ The parameter change to promote this because the subject of the incorporation of SPO4 is a store process of XPP operates in the direction where SPO4 of the treated water is basically reduced. (..treatment as high and calibration ..sensitivity.. object for qPP to change speed of this process directly among these easily..Figure A6. 7)。 ・ Because the speed of this process decreases as the XPP /XPAO ratio rises and it approaches KMAX, (..large in the uptake situation of SPO4 KMAX, too.. ..the influence... Figure A6. 10)。However, you should not change greatly, except when the contribution of GAO is incorporated by parameter value because the default value of this parameter is comparatively thought to be a parameter with high generality by descending on the basis of the measurement data. ・ μPAO comparatively shows big sensitivity, and on the other hand, the amount of XPAO in the faction increases simply even when this is increased from the interaction between the above-mentioned processes and the SPO4 density of the treated water doesn't decrease as a parameter involved in the proliferation of XPAO. Figure A6. 8In ..drinking.. [rei], as the influence when μPAO is improved, XPHA in this tank latter part becomes the limiting factor of the XPP store process, and XPHA is a result of the SPO4 density's rising in front of a conceited tank that remains comparatively though the SPO4 uptake rate increases in steps though it is an increase of XPAO finally. ・ Because bPAO changes the autolysis speed of XPAO, the amount of XPAO is changed without changing the competition between the above-mentioned two processes directly. Therefore, (..reducing this parameter.. ..the SPO4 density of the treated water when the amount is 439 Appendix 6Sensitivity analysis example increased XPAO (Differ from μPAO).. ..the change into the decreasing direction... Figure A6. 9)。 ・ ηNO3 and P are typical as the parameter that influences the behavior of XPAO under the anoxic condition. However, small (..the exclusion of the denitrification actually of the progressing first division in hypoxia tank because of there is room in the capacity of the hypoxia tank in the condition of this discussion.. ..it is the impact combination of that... Figure A6. 12)。 16 2.5 -40% -20% default +20% +40% 14 12 [mg/L] 10 PO4 S 8 6 4 XPAO 2.0 XH 1.5 PO4_eff 1.0 Element variable ratio of a function of change 0.5 PO4_ana 0.0 2 -0.5 0 1 2 3 4 5 6 7 8 9 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 Reactive tank division ParameterVariable ratio of a function of change (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 2Sensitivity analysis result according to phosphorus removal (Analysis object: Kh) 16 3.0 -40% -20% default +20% +40% 14 SPO4 12[mg/L] 10 8 6 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division (a) SPO4 profile 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change (b) Variable ratio of a function of change of element Figure A6. 3Sensitivity analysis result according to phosphorus removal (Analysis object: KX) 440 Appendix 6Sensitivity analysis example 16 3.0 -40% -20% default +20% +40% 14 12 10 8 PO Element 1.0 variable ratio of a function of change 0.5 2 0.0 -0.5 0.2 0 2 3 4 5 6 7 8 PO4_eff PO4_ana 1.5 4 1 XH 2.0 6 S XPAO 2.5 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 Reactive tank division ParameterVariable ratio of a function of change (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 4Sensitivity analysis result according to phosphorus removal (Analysis object: ηfe) 16 3.0 -40% -20% default +20% +40% 14 12 [mg/L] 10 PO4 8 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 6 S 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change Reactive tank division (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 5Sensitivity analysis result according to phosphorus removal (Analysis object: QPHA) 16 3.0 14 -40% -20% default +20% +40% 12 [mg/L] 10 PO4 S 8 6 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 1.0 variable ratio of a function of change Element 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change 441 Appendix 6Sensitivity analysis example (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 6Sensitivity analysis result according to phosphorus removal (Analysis object: Qfe) -40% -20% default +20% +40% 16 14 12 [mg/L] 10 PO4 S 3.0 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 8 Element 1.0 variable ratio of a function of change 6 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change Reactive tank division (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 7Sensitivity analysis result according to phosphorus removal (Analysis object: QPP) 16 -40% -20% default +20% +40% 14 SPO4 12 [mg/L] 10 8 XPAO XH PO4_eff PO4_ana 3.0 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 6 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division (a) SPO4 profile 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change (b) Variable ratio of a function of change of element Figure A6. 8Sensitivity analysis result according to phosphorus removal (Analysis object: μPAO) 442 Appendix 6Sensitivity analysis example 16 3.0 -40% -20% default +20% +40% 14 12 10 8 PO4 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 6 S 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change Reactive tank division (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 9Sensitivity analysis result according to phosphorus removal (Analysis object: BPAO) 16 3.0 -40% -20% default +20% +40% 14 12 [mg/L] 10 PO4 S 8 6 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division (a) SPO4 profile 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change (b) Variable ratio of a function of change of element Figure A6. 10Sensitivity analysis result according to phosphorus removal (Analysis object: KMAX) 443 Appendix 6Sensitivity analysis example 16 3.0 -40% -20% default +20% +40% 14 12 [mg/L] 10 8 PO4 6 S XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 Element 1.0 variable ratio of a function of change 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 Reactive tank division ParameterVariable ratio of a function of change (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 11Sensitivity analysis result according to phosphorus removal (Analysis object: KPHA) 16 3.0 14 12 [mg/L] 10 PO4 S 8 -40% -20% default 2.5 +20% +40% 1.5 XPAO XH PO4_eff PO4_ana 2.0 1.0 variable ratio of a function of change Element 6 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division (a) SPO4 profile 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change (b) Variable ratio of a function of change of element Figure A6. 12Sensitivity analysis result according to phosphorus removal (Analysis object: ηNO3 and P) 444 Appendix 6Sensitivity analysis example 16 3.5 -40% -20% default +20% +40% 14 SPO4 12[mg/L] 10 8 6 2.5 2.0 1.5 Element variable ratio of a function of change 1.0 4 0.5 2 0.0 -0.5 0.2 0 1 2 3 4 5 6 7 8 9 XPAO XH PO4_eff PO4_ana 3.0 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 Reactive tank division ParameterVariable ratio of a function of change (a) SPO4 profile (b) Variable ratio of a function of change of element Figure A6. 13Sensitivity analysis result according to phosphorus removal (Analysis object: YPO4) 16 3.0 14 -40% -20% default +20% +40% 12 [mg/L] 10 PO4 S 8 XPAO XH PO4_eff PO4_ana 2.5 2.0 1.5 1.0 variable ratio of a function of change Element 6 0.5 4 0.0 2 -0.5 0.2 0 1 2 3 4 5 6 7 Reactive tank division (a) SPO4 profile 8 9 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 -1.0 ParameterVariable ratio of a function of change (b) Variable ratio of a function of change of element Figure A6. 14Sensitivity analysis result according to phosphorus removal (Analysis object: YPHA) 445 Appendix 6Sensitivity analysis example 4.Sensitivity analysis on time varying of inflow loadError! Reference source not found. (1)Outline The influent quantity and the water quality have changed timewise in the sewage plant of the reality, and the point even where should reflect this in the simulation is an important concern that controls necessary data of the simulation. It is evident that the influent quantity and the water quality data in equal Thymus vulgaris span are at least needed when it is necessary to expect the water quality variation at the time level. However, if the water quality at the average level of the day is predictable according to the simulation purpose of use and method of the calibration, it is enough. For that case, it is important in the business for the inflow condition data at the time level to be needed still or to understand the point enough if the inflow load at the average level of the day is understood. Then, it introduces the result of examining the influence that the size of the time varying of the influent quantity and the water quality gives the simulation result in this discussion for virtual reluctance-hypoxia-conceited method facilities. (2)Review method Object facilities and process model (..using facilities parameter and process model of facilities A (conceited reluctance-hypoxia-method) used by discussion to propodus..Table A6. 1)。 How to give influent quantity and water quality variation Varying conditions of the influent quantity and the water quality in a day were set by the following policies. ・ Influent quantity and the water quality is mean both (of the day. Table A6. 1The case where the time varying is not given was assumed to be "Basic condition" by using). This is "Error! Reference source not found.Error! Reference source not found.It is identical with the basic condition of this facilities in". ・ Various conditions of giving the time varying of the volume of water and the water quality within the range of 1.2-3.0 compared with the change were set to the basic condition. "Change ratio" was defined here as a ratio of the day maxima and the day mean. ・ The given variability patternFigure A6. 15It is as [ni] is illustrated. In the one 446 Appendix 6Sensitivity analysis example that a typical change of the amount of influent in the sewage plant in our country was assumed, it has the peak in two places (the morning and nighttime). ・ All conditions made a total inlet flow each day of influent and each water quality "Basic condition" and identical. This means the flow rate weighted average value during a day is equal of each water quality. 447 Appendix 6Sensitivity analysis example ・ The same one was used for the variability pattern of the volume of water and the water quality. This reflected seeing the tendency that each element density in influent rises, too in an actual sewage plant at time zone with a lot of influent quantities. ・ When the water quality variation was given, the same variability pattern of all components except biomass element (XPAO ,XAUT ) and alkalinity (SALK ) other than XH was given. ・ When the time varying is given to the influent quantity, the sending back dirt flow rate has been changed in proportion to the influent quantity in consideration of a general operating condition of a real sewage plant. On the other hand, it pulled out and the constancy of the internal circulation flow rate and dirt regardless of the change of the inflow load was other operating conditions like the DO density etc. of a flow rate and a conceited tank. Calculation condition As for the calculation condition in the simulation, it is identical with the discussion to propodus having given the time varying to the inflow condition and the sending back dirt flow rate. 変動比=1.0 3 変動比=1.4 対日平均比 [-] 変動比=2.0 変動比=3.0 2 1 0 0 6 12 18 24 Time [h] Figure A6. 15Time varying pattern of influent quantity and water quality (example of 1.4, 2.0, and change ratio =3.0) 448 Appendix 6Sensitivity analysis example (3)Result of review Influence on nitrification The influence that the time varying of the influent quantity and the water quality gives to the simulation result of the SNH4 density of the treated water (reactive tank end division)(day mean)Figure A6. 16[Ni] was shown. ・ The SNH4 density of the treated water increases as the change of the influent quantity grows. However, the influence is extent in the condition that the change ratio falls below 2.0 that can be disregarded. ・ The tendency to the change of the influent quality similar to the volume of water is seen. Moreover, the size of the influence of the water quality variation is equal to the case to give the same change ratio by the volume of water as long as it sees in the SNH4 density of the treated water. ・ The influence of both is added when the change is given to both the volume of water and the water quality, and the SNH4 density increases further. ・ When the time varying of the density of treated water SNH4 is seen, (Figure A6. 18Volume of water..water quality..change..give..heavy duty..time zone..inflow..as it is..unexpectedly defeat..expected system effluent concentration..appear..day..average..water quality..improve..understand..natural..treated water..remain..reaction..tank..density..decrease.Figure A6. 17)。This suggests that there not be qualitative difference under the influence that the change of the volume of water and the water quality gives. ・ Figure A6. 18As for SNH4 of the treated water, the same behavior as the case to make other element densities change also, too is shown, and it is understood that SNH4 is a subject as the factor affecting of the above-mentioned water quality variation though the simulation result that gives the time varying only to SNH4 of the [niha] influent is shown, too. 449 Appendix 6Sensitivity analysis example 4 There is no water quality variation. Water quality change ratio: 1.4 Water quality change ratio: 2.0 Water quality change ratio: 3.0 3 NH4 S 2 Treated water 1 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 16Influence of influent quantity and water quality variation on SNH4 density of treated water (day mean) 450 Appendix 6Sensitivity analysis example 60 50 [mg/L] 40 AUT X 30 20 There is no water quality variation. Water quality change ratio: 1.4 10 Water quality change ratio: 2.0 Water quality change ratio: 3.0 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 17Influence of influent quantity and water quality variation on XAUT density of reactive tank 7 4 6 [mg/L] NH4 Only the volume(3.0) of water change 5 3 4 (3.0) variation Only the water quality 2 Variability pattern (average toward Japan ratio) There is no change. S 3 Treated 2 water 1 1 SNH4Only the change (3.0) Variability pattern 0 0 0 6 12 18 24 Time [h] Figure A6. 18Time varying of density of treated water SNH4 when volume of water and water quality variation are given Influence on denitrification The influence that the time varying of the influent quantity and the water quality gives to the simulation result of the SNO3 density of the treated water (day mean)Figure A6. 19[Ni] was shown. ・ The influence of the change of both the volume of water and the water quality is not so seen about the SNO3 density of the treated water. ・ But..conceited..tank..nitrification..formation..change..internal Appendix 6Sensitivity analysis example circulation..ratio..change..this..discussion..internal rate..constant..assume..variation in 451 circulation..flow flows..give..condition..internal circulation..ratio..time..change..conceited..tank..steps..denitrification..amount..ch ange..hydrolysis..amount..change..originate..interact..result. It doesn't mean the inflow workload fluctuation doesn't influence the simulation result concerning the denitrification. 452 Appendix 6Sensitivity analysis example 8 7 [mg/L] 6 NO3 S 5 4 3 Treated 2 There is no water quality variation. Water quality change 1.4 1 Water quality change 2.0 Water quality change 3.0 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 19Influence of influent quantity and water quality variation on SNO3 density of treated water (day mean) 800 700 600 [mg/L] 500 400 XH 300 200 There is no water quality variation. Water quality change 1.4 100 Water quality change 2.0 Water quality change 3.0 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 20Influence of influent quantity and water quality variation on XH density of reactive tank 453 Appendix 6Sensitivity analysis example 10 4 [mg/L]8 3 Only the volume(3.0) of water change NO3 6 Japan (3.0) ratio) Only thetoward water quality variation 2 Variability pattern (average S 4 Treated 2 There is no change. 1 0 Variability pattern 0 0 6 12 18 24 Time [h] Figure A6. 21Time varying of density of treated water SNO3 when volume of water and water quality variation are given 454 Appendix 6Sensitivity analysis example ・ Actually, when the time varying of the SNO3 density of the treated water is seen, (..seeing the clear variability pattern... Figure A6. 21)。 ・ ..XH density of reactive tank.. ..remarkable influence of inflow workload fluctuation.. (..not seeing... Figure A6. 20)。 Influence on phosphorus removal The influence that the time varying of the influent quantity and the water quality gives to the simulation result of the SPO4 density of the treated water and the reluctance tank (day mean)Figure A6. 22、Figure A6. 23[Ni] was shown. ・ The SPO4 density of the reluctance tank and the treated water shows different behavior to the time varying of the influent quantity and the water quality. In the same change ratio, it is larger the impact combination about the influent quantity overwhelmingly. ・ The tendency to which SPO4 burst size in the reluctance tank increases when the time varying of the influent quantity grows and the SPO4 density of the treated water decreases is seen. ・ SPO4 burst size of the reluctance tank decreases and the SPO4 density of the treated water increases for the time varying of the influent quality. ・ The change of the amount of the inflow organic loading is an important affector, and SPO4 burst size of the reluctance tank increases for the change of the influent quality by the time zone of the heavy duty to which the organic loading increases. ・ On the other hand, it is necessary to consider the change of real HRT of a reactive tank in addition to this for the change of the influent quantity. The amount of the inflow organic loading becomes the result of the counterbalance of both each other because real HRT of the reluctance tank shortens while it is large at the time zone of the heavy duty. When the time varying situation of the SPO4 density of the reluctance tank is seen, (in the condition with a large change of the amount of influent ..long real HRT of the reluctance tank.. ..the understanding of the SPO4 discharge by time zone of the becoming it low load excellence... Figure A6. 26)。 ・ Because the hypoxia tank latter part contributes to the biological phosphorus removal as a reluctance tank in the facilities condition used by this discussion besides the behavior of such a reluctance tank, the residual situation of SNO3 in the hypoxia tank also influences. Appendix 6Sensitivity analysis example 455 456 Appendix 6Sensitivity analysis example 1.0 [mg/L] 0.8 PO4 0.6 S 0.4 Treated 0.2 There is no water quality Water variation. quality change 1.4 Water quality change 2.0 Water quality change 3.0 0.0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 22Influence of influent quantity and water quality variation on SPO4 density of treated water (day mean) 16 [mg/L] 14 12 PO4 S 10 8 6 Reluctance tank 4 There is no water quality Water variation. quality change 1.4 2 Water quality change 2.0 Water quality change 3.0 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 23Influence of influent quantity and water quality variation on SPO4 density of reluctance tank (day mean) 457 Appendix 6Sensitivity analysis example 400 350 300 [mg/L] 250 PAO X 200 150 100 There is no water quality Water variation. quality change 1.4 50 Water quality change 2.0 Water quality change 3.0 0 1.0 1.5 2.0 2.5 3.0 [-] change ratio Volume of water Figure A6. 24Influence of influent quantity and water quality variation on XPAO density of reactive tank 4 4 [mg/L] 3 3 PO4 S Only the volume(3.0) of water change (3.0) ratio) Japan Only thetoward water quality variation 2 Variability pattern (average 2 There is no change. Treated water 1 1 0 Variability pattern 0 0 6 12 18 24 Time [h] Figure A6. 25Time varying of density of treated water SPO4 when volume of water and water quality variation are given 40 4 [mg/L] 35 30 PO4 S 3 25 Only the volume(3.0) of water change (3.0) Japan variation ratio) Only the toward water quality 2 Variability pattern (average 20 15 tank Reluctance 10 There is no change. 1 5 0 0 0 6 12 Time [h] 18 24 Variability pattern 458 Appendix 6Sensitivity analysis example Figure A6. 26Time varying of density of reluctance tank SPO4 when volume of water and water quality variation are given Appendix 6Sensitivity analysis example 459 Summary of influence on treated effluent quality Figure A6. 27As for the change of both the influent quantity and the water quality, in the range where the change ratio falls below 2.0, treated effluent quality (SNH4 ,SNO3 ,SPO4 ) is [ni] equal to the basic condition in the facilities condition used by this discussion as shown (That is, there is no big influence in the simulation result even if the average volume of water of the day and the water quality are used as input data). However, because the acquisition of the time varying data is easy, the influent quantity can be said it should the reflection in the simulation regardless of the size of the change. On the other hand, after the change realities are confirmed (If it is possible, the evaluating impact like this discussion is executed), it is necessary to decide the use data though it seems that you may often use the data of the day average about the influent quality because the influence equal with the volume of water change may be given to the simulation result of nitrification about the time varying of the density of the ammoniacal nitrogen. 3 [-] (a) There is no volume of water change. 2 1 Variable 3 ratio of a function of change to basic condition (b) Volume of water 2.0change ratio = 2 1 0 NH4-N NO3-N Water quality change It is ratio not. 1.4 PO4-P 2.0 3.0 Figure A6. 27Variable ratio of a function of change of average treated effluent quality of day when influent quantity and water quality variation are given 460 Appendix 6Sensitivity analysis example Bibliography 6A) Hiroshi Itokawa [**] and Takao Murakami: Response of activated sludge model to a variety of inflow workload fluctuations, the 42nd drainage research symposium lecture collections, and pp.112-114,2005. 6B) Japan Sewage Works Association: Japan Sewage Works Association and sewerage facilities plan, design principle, and explanation-2001 edition -2001. 6C) Brun,R., Kuhni,M., Siegrist,H. Gujer,W. and Reichert,P.: Practical identifiability of ASM2d parameters- systematic selection and tuning of parameter subsets. Wat.Res., Vol.36, pp.4113-4127, 2002. 6D) Reichert,P.: AQUASIM- a tool for simulation and data analysis of aquatic systems. Wat.Sci.Tech., Vol.30, No.2, pp.21-30, 1994. Appendix 7Example of oxidation ditch process model development 461 Appendix7Discussion case with process model of oxidation ditch method 1.Outline When the simulation by the activated sludge model is attempted in our country for [okishide-shondei;cchi] (OD) method with a lot of introduction results, the method for constructions of the following process models become problems. ・ The inclination of DO and the density of dirt might be caused in the direction of depth and the direction of the width of the waterway according to the kind, the ability, and the operational method of the diffused air and the stirrer though a reactive tank essentially has problem data conversion of the plug flow type. It is necessary to discuss an appropriate modeling methodology of problem data conversion in the tank. ・ The influent quantity is done and driving that installs the process of decreasing the output of the diffuser (cycle) is done generally centering on the case where the oxygen supply ability of the diffuser becomes relatively excessive small for the process capability and in the case of, etc. to do the denitrification. The flow rate in the tank will change according to the change in the output when the diffuser bears the stir. Moreover, influent and the sending back dirt will flow in while dirt's the process of completely stopping all diffusers according to facilities might be incorporated, and it having subsided to the belt during that time in the tank. Thus, it is necessary to discuss the process model that is expressible of the amount of the oxygen supply corresponding to the operational method of the diffuser and the change in the flow rate in the tank. ・ In the regular processing method, it is 3,000-5,000 as for the MLSS density of a reactive tank Setting it higher than the active sludge process is usual about mg/L and usually. Therefore, it is designed so that the scale of the final sedimentation tank may grow. Therefore, it can be said that the dwell time of the supernatant fluid water and dirt in the final sedimentation tank becomes long, and the possibility that the biological response there gives the influence that cannot be disregarded to the behavior of the entire processing process is higher than other processing mode. An appropriate modeling methodology to expect the biological 462 Appendix 7Example of oxidation ditch process model development response in the final sedimentation tank is necessary. One side, and in the OD method, the diversitys of the kind and the spec etc. of the shape of a reactive tank and the final sedimentation tank to the process capability, parameters, and diffusers are remarkable and are the smalls one of the most advanced methods in various processing mode adopted in our country by standardization. This means the possibility that the process model constructed for a certain facilities can be generally used is high. It introduces the case that discusses each element of the process model of the OD method from such an aspect here on the basis of the data that collects chiefly in real facilities. It is thought that the process model found here is useful to provide the illustrative example of the process model construction work besides use as the basic plan when other similar facilities are modeled can be expected. Here, OD method facilities (of the horseshoe type to which two spindle type diffusers with most numbers of adoptions in our country are distributed. Figure A7. 1) was made the main object. ・ Modeling methodology of reactive tank ・ Modeling methodology of oxygen supply corresponding to change in driving strength of diffuser ・ Modeling methodology of flow rate in reactive tank corresponding to change in driving strength of diffuser ・ Modeling methodology of dirt subsidence when diffuser is stopped Appendix 7Example of oxidation ditch process model development 463 [Okishide-shondei;cchi] ( OD) Treated water Finality Sedimentation pond Influent (crude sewage) Excess sludge Sending back dirt Spindle type diffuser Figure A7. 1Reactive tank shape and facilities composition of OD method facilities made object of process model discussion 464 Appendix 7Example of oxidation ditch process model development 2.Discussion about modeling methodology of reactive tank (1)Outline It is general to be realistic not to model as a plug flow type reactor no numerical calculation, to describe this according to the complete mixing [souretsu] model, for the mixture to circulate to the foremost tank from the final tank, and to express the no terminal waterway though problem data conversion in OD is extremely near the plug flow as already stated. Because computing time increases according to it, it is ideal to set minimum number of tanks within the range where it doesn't influence the prediction result though it becomes problem data conversion near the plug flow to increase the number of tanks that composes the model. However, a necessary number of tanks is various to the one for the number of tanks that is expected the difference depending on the kind, the output, the number, arrangement, and the loading condition etc. of capacity and the diffuser of a reactive tank, and used actually in the case where OD of the full scale is modeled in foreign countries to exceed 20 tanks from one tank. 7B) ~7K) 。 An enough discussion case is about the OD method facilities in our country, and on the other hand, the majority of a no bur and the above-mentioned facilities condition are standardized and there is a possibility that a generality and high model can comparatively be found because facilities that are designed and driven on the basis of this are a lot of. Then, the modeling methodology of OD was discussed for typical OD method facilities in our country. Because the generation situation of the water quality in OD and the density gradient such as dirt was investigated before this, it also introduces the result. (2)Data collection It is necessary to understand how to cause the density gradient of the solid and the water quality in the tank when modeling OD is discussed. For instance, it is extent to be able to be necessary the model who is appropriately expressible of it, and to disregard the solid in the tank and the density gradient of the water quality if the area where the DO density is low is caused by a part of division in a reactive tank, and the entire tank can be considered to be one complete mixing tank in the aerification worker degree. Then, the generation situations of the density gradients such as solids and DO were investigated for the real OD method facilities under operation. Density gradient investigation of DO and solid in waterway section 465 Appendix 7Example of oxidation ditch process model development DO in the waterway section in each place in OD and the distribution of the solid concentration were investigated for the real OD method facilities under operation. < object facilities > The outline of the object facilities is as follows. ‒ Reactive tank shape:Horseshoe type ‒ Capacity of reactive tank: 1,650 m3 ‒ Diffuser form kilowatt-hour)×2 and the number:Spindle type (Declared power: 15 466 Appendix 7Example of oxidation ditch process model development ‒ Diffuser ratings oxygen supply ability (per one): 599 kgO2/d < search procedure and content > Five-place (A-E in the OD tank by three conditions to change the operational method of two diffusers;Figure A7. 2DO and the solid concentration were measured by). In each measured point, nine point (of the passage section. Figure A7. 3It measured it with the DO meter and dirt photographic densitometer in)(Only the distribution of the direction of depth of the center part of the waterway was measured in D and E that placed the straightening vane). The operational method of the diffuser assumed to be a measuring object is as follows. ‒ Condition 1 (normal operation): Main machine: High speed driving (80%) and accessory: High speed driving (80%) ‒ Condition 2 (one slow running): Main machine: High speed driving (80%) and accessory: Slow running (33%) ‒ Condition 3 (two slow running): Main machine: Slow running (33%) and accessory: Slow running (33%) < finding > It is of each as for the measurement result of DO and the solid concentration. Table A7. 1、 Appendix 7Example of oxidation ditch process model development 467 Table A7. 2[Ni] was shown. ・ In each operating condition, the inclination of remarkable DO and the solid concentration is not caused in the waterway section. ・ The difference is seen about the DO density though the solid concentration is roughly equal comparing a different measurement part. Sedimentation pond E C D F B A +Y Influent Sending back dirt Aerator +X 4-15.Measurement point outline Figure Figure A7. 2Measurement part in tank in density gradient investigation in waterway section 468 Appendix 7Example of oxidation ditch process model development c b Flow direction a +Y 1 2 +Z +X 3 4-17.Measurement point outline Figure c b a 1950 300 1950 300 +X 500 1 750 +Z 2 900 3 250 b-2 is driven: - Reading point when non-stationary - Flow direction: From the interior to the this side side 4-16.Measurement point outline Figure Figure A7. 3Measured point in waterway section in density gradient investigation in waterway section Table A7. 1DO concentration measurement result in density gradient investigation in waterway section Condition 1 (normal operation) Condition 2 (one slow running) Condition 3 (two slow running) A a b c A a b c A a b c 1 2 0.25 0.14 0.10 0.33 0.22 0.13 0.07 0.05 0.14 0.10 0.25 0.15 0.10 1 2 0.10 0.19 1 2 0.10 0.07 0.06 469 Appendix 7Example of oxidation ditch process model development 3 0.19 0.12 0.10 3 0.20 0.15 0.11 3 0.08 0.07 0.06 B a b c B a b c B a b c 1 2 3 0.12 0.10 0.10 0.15 0.09 0.10 0.08 0.07 0.10 0.10 0.15 0.09 0.08 0.09 0.08 0.07 0.11 0.09 0.10 0.10 0.08 0.10 1 2 3 0.07 0.10 1 2 3 0.08 0.07 0.10 C a b c C a b c C a b c 1 2 3 0.40 0.94 0.33 0.25 0.25 0.24 0.20 0.20 0.84 0.28 0.23 0.22 0.24 0.27 0.15 0.20 0.60 0.51 0.25 0.22 0.25 0.16 1 2 3 0.25 0.65 1 2 3 0.20 0.16 0.20 D a b c D a b c D a b c 1 2 3 - 0.50 - 0.10 - 0.10 - - - 0.09 - - 0.09 - - 0.46 - - 0.07 - 1 2 3 - 0.49 1 2 3 - - - 0.08 - E a b c E a b c E a b c 1 2 3 - 0.45 - - 0.11 - 0.10 - 0.50 - - 0.10 - - 0.09 - - 045 - - 0.07 - 1 2 3 - - 1 2 3 - 0.08 - 470 Appendix 7Example of oxidation ditch process model development Table A7. 2Solid concentration measurement result in density gradient investigation in waterway section Condition 1 (normal operation) Condition 2 (one slow running) Condition 3 (two slow running) A a b c A a b c A a b c 1 2 3 2,470 2,540 2,540 2,140 2,510 2,350 2,470 2,470 3,200 2,670 3,600 2,530 2,500 2,540 2,540 2,500 2,540 2,940 2,590 2,940 2,450 2,540 1 2 3 2,410 3,200 1 2 3 2,610 3,130 2,530 B a b c B a b c B a b c 1 2 3 2,540 2,610 2,470 2,540 2,540 2,540 2,470 2,540 2,610 2,510 2,540 2,500 2,610 2,510 2,470 2,500 2,610 2,610 - 2,540 - - 1 2 3 2,470 2,610 1 2 3 2,500 2,670 - C a b c C a b c C a b c 1 2 3 0.40 0.94 0.33 0.25 0.25 0.24 0.20 0.20 0.84 0.28 0.23 0.22 0.24 0.27 0.15 0.20 0.60 0.51 0.25 0.22 0.25 0.16 1 2 3 0.25 0.65 1 2 3 0.20 0.16 0.20 D a b c D a b c D a b c 1 2 3 - 2,530 - - 2,410 - 2,470 - 2,540 - - 2,410 - - 2,510 - - 2,540 - - 2,610 - 1 2 3 - - 1 2 3 - 2,620 - E a b c E a b c E a b c 1 2 3 - 2,470 - - 2,540 - 2,540 - 2,500 - - 2,540 - - 2,470 - - 2,510 - - 2,540 - 1 2 3 - - 1 2 3 - 2,530 - Density gradient investigation of DO and dissolubility water quality parameter in direction of length of waterway The time varying situation of a dissolubility water quality parameter according to DO and nitrogen and Rin was investigated in each place in OD for the real OD method facilities under operation. < object facilities > The outline of the object facilities is as follows. ‒ Reactive tank shape:Horseshoe type ‒ Capacity of reactive tank: 1,329 m3 ‒ Diffuser form and the number:Spindle type (Declared power: 11 Appendix 7Example of oxidation ditch process model development kilowatt-hour)×2 ‒ Diffuser ratings oxygen supply ability (per one): 439 kgO2/d 471 472 Appendix 7Example of oxidation ditch process model development < search procedure and content > Four-place in OD tank (① -④;Figure A7. 4And, the mixture obtaining water of 24 2h pitch × h was done, and NH4-N, NO2-N, NO3-N, and the PO4-P density were measured in the part immediately after the outflow from this tank. The measurement of the DO density with the DO meter was also executed at ..adoption.. [mizu]. < finding > The measurement result of each water quality itemFigure A7. 5[Ni] was shown. ・ A remarkable difference by the measurement part is not seen in NH4-N, NO2-N, NO3-N, and the PO4-P density. ・ The difference of the density of the clear is seen by [mizu] ..adoption.. part about the DO density. DO decreases from the diffuser in ② and ④ with the distances comparatively, and it is understood that it is a level not to be able to disregard the oxygen consumption rate in the flowing process in the tank compared with the flow rate in the tank. The reactive tank model's discussion The modeling methodology of OD was discussed on the basis of the above-mentioned finding by the following policies. ・ The complete mixing [souretsu] model is used. ・ You may consider that the solid concentration is uniform in a reactive tank as long as the operating condition to which dirt subsides is not set. That is, there is no necessity for building in the phenomenon of the subsidence etc. in a usual driving. ・ Because a clear difference is seen by the part in the DO density in the direction of the length of the waterway, it is improper to model the direction of the length of the waterway in one complete mixing tank. ・ The DO density gradient in the direction of the width of the waterway and the direction of depth is the extent that can be disregarded. Therefore, there is no necessity for dividing the waterway section into two or more tanks. ・ A virtual tank of the small capacity is installed in the part (influent, sending back dirt inflow part, diffuser installation place, and treated water outflow part) with the inflow going out of the material in consideration of the plug flow type characteristic. There is no necessity assumed to be place's independent tank Appendix 7Example of oxidation ditch process model development because it doesn't consider the inclination of the solid concentration however. 473 474 Appendix 7Example of oxidation ditch process model development Influent and sending back dirt ④ Spindle rotor (main machine) ① ② ③ Rise To the final sedimentation tank Excess sludge Figure A7. 4Measurement part in density gradient investigation of flow direction 1 ① ③ Outflow 0.8 -N [mg/L] 0.6 4 NH 16 14 ② ④ 12 -N [mg/L] 10 0.4 3 NO 0.2 8 6 4 ① ③ Outflow 2 0 0 9 11 13 15 17 19 21 23 1 3 5 7 9 9 11 13 15 17 19 21 23 Time [h] 1 3 5 7 9 1 3 5 7 9 Time [h] (a) NH4-N (b) NO3-N 5 5 ① ③ Outflow 4 -P [mg/L] ② ④ ① ③ Outflow 4 DO [mg/L] 3 3 4 PO ② ④ 2 2 1 1 0 ② ④ 0 9 11 13 15 17 19 21 23 Time [h] (c) PO4-P 1 3 5 7 9 9 11 13 15 17 19 21 23 Time [h] (d) DO Appendix 7Example of oxidation ditch process model development 475 Figure A7. 5Time variance of each water quality in density gradient investigation of flow direction 476 Appendix 7Example of oxidation ditch process model development Because detailed water quality profile data in OD in real facilities was not obtained when the number of tanks of complete mixing [souretsu] models is discussed, virtual facilities condition that seems to be typical it (here. Table A7. 3It was assumed that the number of tanks that did not influence the calculation result was found by setting), and doing the simulation that changed the number of division tanks of directions of the length of the waterway on the process model within the range of 1-12. The resultFigure A7. 6It is as showing [ni]. ・ The number of division tanks receives and the number of tanks influences the calculation result of the treated effluent quality strongly in three or less. Therefore, such small number of tank division is improper. ・ On the other hand, the number of division tanks is equal and the calculation result is equal in the range of 4-12. Table A7. 3Simulation condition of virtual facilities used to discuss number of composition tanks of reactive tank models Facilities condition OD capacity 1600 m3 Capacity of the final sedimentation pond Diffuser ability 700 m3 1300 m3/d Influent quantity Inflow condition SF :11 mg/L、SA :5 mg/L、SNH4 :21 mg/L SNO3 :0 mg/L、SPO4 :1 mg/L、SI :11 mg/L XI : 70mg/L、Xs : 148mg/L、XH :31 mg/L Influent quality Dirt sending back ratio Operating condition and others Amount sludge of excess Diffuser driving cycle Water temperature Calculation condition Biological response model Numerical calculation method Initial condition Calculation days 600 KgO2/d×2 0.8 22 m3/d High speed (80%): 1h and the stop: 2h of 1h is assumed to be a basic cycle, and it changes according to the load. 16℃ ASM2d Calculation given repeating variability data during a day Stationary solution when number of composition tanks is made eight tanks 120 days Appendix 7Example of oxidation ditch process model development 4.0 477 4.0 NH4-N NOx-N PO4-P 3.5 3.0 2.5 Element density 3.0 2.5 density mg/L Element 2.0 2.0 1.5 1.5 1.0 1.0 0.5 0.5 0.0 1 2 Three (rise3 soon) 4 5 6 8 12 Number of division tanks steps in OD tank T-N NH4-N T-P 3.5 0.0 1 2 Three (rise3 soon) 4 5 6 8 12 Number of division tanks steps in OD tank Figure A7. 6Calculation result of treated effluent quality in various conditions to change number of composition tanks of reactive tank models It tempers this result with room and the inflow part of influent and the sending back dirt and the outflow parts of the treated water : in consideration of compatibility when differing. Figure A7. 7The reactive tank model who consisted of eight shown [ni] tanks decided to be assumed to be basic. ・ The capacity of influent, the sending back dirt inflow tank, the diffuser installation tank (two tanks), and the treated water outflow tanks is each 3%, is 5%(×2), 5%, and smalls of a total tank capacity among eight tanks where the [souretsu] model is composed. ・ Four tanks of the remainder correspond to the straight-line portion of OD of the horseshoe type, and account for 21% of a total tank capacity respectively. It can be said that these are subjects of the place of the biological response. ・ The flow rate in the tank is 0.1-0.25 when based on the following flow rate models It is a range of m/s. This is 1.9-4.7 as each four tank above real HRT It is corresponding in min. 478 Appendix 7Example of oxidation ditch process model development Sending back dirt Influent OD1 3% OD2 5% OD3 21% OD4 21% Oxygen OD5 21% OD6 21% OD7 5% OD8 5% Oxygen Final sedimentation [He] tank - Percent table[Shime] ..drinking.. numerical value shows (totalthe capacity capacity ratio)。 of each tank. Figure A7. 7Tank composition of reactive tank model of OD 479 Appendix 7Example of oxidation ditch process model development 3.Discussion about modeling methodology of oxygen supply (1)Outline As for the spindle type diffuser used in the OD method facilities in our country, driving that can usually adjust the cycle by the inverter, intends the control of nitrification and the reduction in the electric power used, and changes the cycle timewise is done actually widely. Enough information is not given about the condition of remarkably decreasing the cycle though the oxygen supply speed can be presumed if this is used (Shimizu) because the performance curve of which the index is the cycle is given to the device (On a usual business, it is considered that there is no oxygen supply and only the stir power is given). Then, the relation between the cycle and the oxygen supply speed in a wide range of the cycle was intended to be found by measuring KLa on two or more conditions to change the operational method of two diffusers (cycle), and analyzing it along with the performance curve of the device in real facilities where the trial run by Shimizu before it used it was done. Describing the oxygen supply by assuming the cycle to be a logical input value to the given diffuser by building this in the process model as an incidental model becomes possible. (2)Data collection Figure A7. 1KLa was measured in the OD method facilities before it used it with equal facilities composition. Object facilities Various conditions of the object facilities are as follows. ‒ Reactive tank shape:Horseshoe type ‒ Capacity of reactive tank: 1,240 m3 ‒ Diffuser form and the number:Spindle type (Declared power: 11 kilowatt-hour)×2 ‒ Diffuser ratings oxygen supply ability (per one): 344 kgO2/d Diffuser operating condition KLa was measured under the following four conditions to change the cycle of two diffusers. 480 Appendix 7Example of oxidation ditch process model development ‒ Condition 1:High speed (44 rpm) + high speed (44 rpm) ‒ Condition 2:High-speed (16 rpm) of (44 rpm) + low speed ‒ Condition 3:High speed (44 rpm) + stop ( 0 rpm) ‒ Condition 4:Low speed (16 rpm) + low speed (16 rpm) - A set cycle is shown about each condition in order of "Main machine + accessory". About two diffusers and the positions of the KLa measurement partFigure A7. 8Refer to [wo]. 481 Appendix 7Example of oxidation ditch process model development - Because the cycle in the declared power is 45 rpm, "High speed" and "Low speed" mentioned here are corresponding to the output of 98 and each 36% against ratings. KLaMeasurement part Main machine Accessory Oxidation [Dei;cchi] Flow rate measurement part Figure A7. 8Position of measurement part of KLa and flow rate and two diffusers Measuring method of KLa Because Shimizu should not filled a reactive tank for the trial run, and the biological response in the tank be considered in this facilities, the unsteady method. source not found.[Niyori] Error! Reference KLa was measured. The DO sensor for the DO concentration measurementFigure A7. 8It set it up in the shown measurement part of [ni]. First of all, a sodium sulfite 110ppm a large amount was added to consume DO in the tank, it stirred with the diffuser in the tank, and DO was 0 It was confirmed to decrease to mg/L. Next, the diffuser was stopped once and after it had been confirmed that the flow rate in the tank decreased enough, it was operated by the cycle in which two diffusers were set. KLa was calculated from the rise rate of DO at that time by following equation. KLa dCO 2 1 CO 2, S CO 2 dt Density..measurement..water temperature..saturation..density. Expression (A7. 1) 482 Appendix 7Example of oxidation ditch process model development In the water temperature when measuring it, the soaking depth of 2-3℃ and the scatter nature rotor ([sabuma-jensu]) is 50mm. 483 Appendix 7Example of oxidation ditch process model development (3)Measurement result and analysis The KLa measurement result and this by four conditions were converted into the oxygen supply speed (It is only called, "Oxygen supply speed" as follows) in the "20℃,DO=0 mg/L" condition. Table A7. 4[Ni] was brought together. ・ KLa decreases greatly when the cycle of the diffuser is decreased up to 36% of ratings, and the oxygen supply of the amount that cannot be disregarded is caused in condition 4 of driving both of the two diffusers in low speeds (About 5% of condition 1 driving both at high speed). ・ (..add as the cycle of two diffusers is simple.. ..cannot the enough expression of the relation to the oxygen supply speed as the index of matched what... Figure A7. 9)。 This suggests that the relation between the cycle and the oxygen supply speed be not linear in one diffuser. ・ Then, the oxygen supply speed per one of the diffusers is presumed from the measurement result, and what plotted along with performance curve of the device data :. Figure A7. 10..going out.. .It is understood that approximating by the straight line is improper when including it to cycle lower area though most straight lines in the area where the cycle is high the dependency at the oxygen supply speed to the cycle. Then, the relation between the cycle (driving strength) and the oxygen supply speed was assumed to be approximate by the 2 secondary type here. Table A7. 4Measurement result at KLa and oxygen supply speed Oxygen supply speed KLa [h-1] kgO2/d * Condition 1 2.14 625 High speed (44 rpm) + high speed (44 rpm) Condition 2 1.27 360 High-speed (16 rpm) of (44 rpm) + low speed Condition 3 1.27 360 High speed (44 rpm) + stop (0 rpm) Condition 4 Low speed (16 rpm) + low speed (16 rpm) - Water temperature =20℃ and correction value to condition of DO=0 mg/L. 0.10 29 484 Appendix 7Example of oxidation ditch process model development Condition 1 700 /日] 600 2 Condition 3 500 Condition 2 Oxygen400 supply..speed..total. 300 200 Condition 4 100 0 0 20 40 60 80 100 Cycle of aeration device (two totals) Rpm Figure A7. 9Simple harmony of cycle of two diffusers and relation to total oxygen supply speed Figure A7. 10Relation between driving strength and oxygen supply speed of one diffuser 485 Appendix 7Example of oxidation ditch process model development (4)The oxygen supply model's discussion Discussion..obtain..diffuser..per..drive..strength..oxygen supply..speed..relation..homotype..device..declared power..differ..large..differ..assume..given..declared power..have..device..oxygen supply..speed..drive..strength..ratings..output..ratio..index..following equation..generalization. RO 2 435.01 f a 91.30 f 2 RO 2,max 344 RO 2 RO 2,max 1.26 f a 0.27 f a 2 Expression (A7. 2) It is RO2 here: oxygen supply speed kgO2/d and RO2 and max: ratings oxygen supply ability kgO2/d of the diffuser and fa: driving strength (The ratings ratio; Having obtained the measurement data is in the range of 0.36-0.98) -. Because the ratings oxygen supply ability of the diffuser is given on the condition of Shimizu, 20℃, and DO=0 mg/L, KLa in this condition can be calculated by following equation. RO 2 10 3 KLa Vaer S O* 2, 20 Expression (A7. 3) Diffuser..installation..division..volume..water temperature..saturation..density. It is KLa ..(.. expression if it calculates to correct the mixture and the water temperature by using this by the method similar to a usual facilities design as for ' Expression (A7. 4)The DO application rate in the mixture at the given water temperature is an expression. Expression (A7. 5)[De] can calculate. K L a' K L a 1.024 (T 20) dS O 2,aer dt K L a' S O* 2,T S O 2,aer Expression (A7. 4) Expression (A7. 5) 486 Appendix 7Example of oxidation ditch process model development SO2 and aer ..here..: DO density mg/L of the diffuser installation division and saturation DO ..KLa 'Mixture correction factor - of water temperature, KLa d-1 that corrects the mixture, and α:KLa and β: mixture correction factor - of saturation DO density and S*O2 and T:.. density mg/L at water temperature T℃. Appendix 7Example of oxidation ditch process model development 487 4.Discussion about modeling methodology of flow rate in reactive tank (1)Outline The flow rate in the tank changes, too, when the cycle is changed so that this may bear the stir (secure of the flow rate) in in addition to the oxygen supply a reactive tank when the spindle type diffuser is used. As a result, it is expected to change DO in a reactive tank and the generation situation of the density gradient of other substrate, and to influence processing status. Then, the flow rate in a reactive tank was also measured in the KLa measurement shown in the preceding clause. The relation to the flow rate in the operational method of two diffusers (cycle) and the tank was arranged by using this, and the appropriate modeling methodology was discussed. Dirt subsides, will pile up in a reactive tank in the condition to stop two diffusers completely, and it be a result of review that shows here simply squeezed to "Expectation of the flow rate". The discussion is added by the next paragraph about the modeling methodology of the condition that dirt piles up. (2)Data collection The flow rate in a reactive tank was measured in the same to what targeted by discussing the preceding clause facilities. Diffuser operating condition The flow rate was measured under the following three conditions to change the cycle of two diffusers. ‒ Condition 1:High-speed (44 rpm) of (43 rpm) + high speed ‒ Condition 2:High speed (41 rpm) + stop ( 0 rpm) ‒ Condition 3:Low-speed (15 rpm) of (14 rpm) + low speed Method of measuring flow rate After driving the diffuser in the setting condition begins, and the flow rate in the tank is steady enoughFigure A7. 8Nine point in passage section (in the shown measurement part of [ni]. Figure A7. 11The flow rate was measured by) one by one. (for X, Y, and Z everyone with the electromagnetic flowmeter of three dimensions it for the measurement. Figure A7. 11Two of the) of the identifying values taken by 1s pitch The mean between min was assumed to be measurements. 488 Appendix 7Example of oxidation ditch process model development (3)Measurement result and analysis Flowing direction in tank in three operating conditions (Figure A7. 11Distribution of the flow rate of the direction of ..drinking.. Y) in the direction of the width of the waterway according to depthFigure A7. 12[Ni] was shown. The flow rate in the direction of the width of the waterway (direction of this figure X) and the direction of depth (direction of this figure Z) is 0.05 It disregarded it here because it was remarkably small compared with the flow rate in m/s or less and the direction of Y. Appendix 7Example of oxidation ditch process model development 489 c b Flow direction a +Y 1 2 +Z +X 3 Figure A7. 11Measured point in passage section in flow rate measurement part ・ As for condition 1-3, a simple mean of the flow rate (It is called, "Total mean flow velocity" at the following) in the measured point in nine places is 0.25 respectively, 0.17, and 0.15 It is m/s. In condition 2 of driving only one compared with condition 1 of high-speed rotating both of the two diffusers and condition 3 to assume two to be a low-speed rotation both, the flow rate has decreased obviously. This is 180 times amount (1,200 m3/d) of the design influent as the amount of the circulation in the tank in this facilities. corresponding of in m3/d a total mean flow velocity in condition 1 ・ However, 0.1 that is the standard of the lowest flow rate that doesn't make dirt subside in all conditions and the measured points It doesn't enter the state that greatly falls below m/s. ・ In the direction of the width of the waterway, (..seeing the tendency that the flow rate grows heading for the direction of corresponding outside of in the time of surroundings part... Figure A7. 12;When the average of the flow rate by all depth is taken about three places of the direction of the width of the waterway, it is 0.17 respectively, 0.26, and 0.31 from the inside to the outside in condition 1 It is 0.14, 0.19, and 0.23 in m/s and condition 2 It is 0.15, 0.16, and 0.17 in m/s and condition 3 Become m/s. )It is a result of it is easy to understand if it thinks the centrifugal force works at the time of surroundings part. The difference of the total mean flow velocity seen between condition 2 and 3 originates chiefly in the 490 Appendix 7Example of oxidation ditch process model development difference of the flow rate outside of the waterway. ・ When the average of the flow rate in the direction of the width of the waterway is taken about each depth, it is 0.26 respectively, 0.26, and 0.23 in condition 1 for the direction of depth It is 0.21, 0.17, and 0.17 in m/s and condition 2 It is 0.16, and is 0.15, and 0.16 in m/s and condition 3 It is m/s. The flow rate in the bottom causes and a flow velocity distribution remarkable like the direction of the width of the waterway is not caused though the small tendency is seen. ・ The number of mean rotations of two diffusers of total mean flow velocity in each operating condition is taken in the axis of abscissas and plotted what :. Figure A7. 13..going out.. .Both show an excellent linear relationship, and are suggesting that the number of mean rotations (output) is assumed to be an index and the flow rate in the tank be predictable. Appendix 7Example of oxidation ditch process model development 491 < azimuth > In case of the above →(direction of Z) Outside → on inside (direction of X) Direction of flowing (direction of y) > 0.4 Height-amount Low-lower Flow0.3rate (+Y) in tank M/sec [Henhiku] 0.2 0.1 0.0 Inside Inside X coordinates (+X) The outside < inside > 0.4 Height-amount Low-lower Flow0.3rate (+Y) in tank M/sec [Henhiku] 0.2 0.1 0.0 Inside Inside X coordinates (+X) The outside < under > 0.4 Height-amount Low-lower Flow0.3rate (+Y) in tank M/sec [Henhiku] 0.2 0.1 0.0 Inside Inside X coordinates (+X) The outside Figure A7. 12Flow velocity distribution in direction of width of waterway in each condition and depth 492 Appendix 7Example of oxidation ditch process model development Condition 3 Condition 2 Condition 1 0.3 0.2rate (+Y) in tank m/second Flow 0.1 y = 0.0034x + 0.1015 R2 = 0.9994 0.0 0 20 40 60 Simple arithmetic average rpm of cycle Figure A7. 13Number of mean rotations of two diffusers and relation to total mean flow velocity (4)The flow rate model's discussion Figure A7. 13It drinks, driving strength of the person in charge of [kan] is assumed to be an index, and what arranged as a predicting that doesn't depend on the declared power of the diffuser again is following equation. It can be said that there is no obstacle even if it generalizes like this expression within this range because in the planning approach of the OD method facilities usually used in our country, the quantity of water to be treated, the capacity of a reactive tank, and the diffuser output are respectively roughly in the proportional connection. It is necessary to note it because regularity cannot be simply applied when the relation between the capacity of a reactive tank and the diffuser specification is greatly different from this. FOD 0.14 f a ,ave 0.10 Expression (A7. 6) It is FOD here: mean flow velocity m/s in a reactive tank and fa and ave: Average driving strength-. As the logical input value of driving strength of the diffuser in building in the process model of regularity, flow rate (..in a reactive tank... Figure A7. 7Describing the amount Appendix 7Example of oxidation ditch process model development 493 of the circulation in case of the [noyouna] [souretsu] model) becomes possible. It can be said that driving strength that is bigger than this is a regular range because there is a possibility that the measurement data is not obtained within the range where average driving strength of two diffusers is 32% or less and the subsidence of dirt is caused, too. 494 Appendix 7Example of oxidation ditch process model development 5.Discussion about modeling methodology of dirt subsidence when diffuser stops (1)Outline When the inflow load volume doesn't especially come up to the designed value, the example of building in the process of completely stopping the device in the run cycle of the diffuser is often seen in the OD method facilities in our country as shown in the above-mentioned. In that case, influent and the sending back dirt will flow in with dirt subsided in the OD tank. It is necessary to understand what kind of water quality changing is causing respectively in the mixed clinical posture in the tank after the diffuser stops, the supernatant fluid water part, and the piling up dirt to model such a process. Then, the investigation was executed in real facilities under operation, and the modeling methodology was discussed on the basis of it. (2)Data collection The tracer experiment after the (b) diffuser had stopped of the measuring water quality in a reactive tank after the (a) diffuser had stopped was executed in the OD method facilities under operation when the process (stop process) where the diffuser stopped completely was built in. Object facilities Various conditions of the object facilities are as follows. ‒ Reactive tank shape:Horseshoe type ‒ Capacity of reactive tank: 1,330 m3 ‒ Diffuser form and the number:Spindle type (Declared power: 11 kilowatt-hour)×2 ‒ Diffuser ratings oxygen supply ability (per one): 439 kgO2/d Measuring water quality after diffuser stops 75 63 immediately after the process beginning against the stop process of min The mixture was collected from the vicinity of the surface and the surface of the water from about 150cm(It is 85cm from the sludge-liquid interface to the direction of depth) in 5 times and 3 places in a reactive tank between after min, and NH4-N and the NOx-N density were measured. Appendix 7Example of oxidation ditch process model development 495 The operating condition when the investigation is executed is as follows. ‒ Influent quantity: 922 m3/d ‒ Sending back dirt flow rate: 1160 M3/d (1.3 compared with sending back) ‒ Depth of reactive tank: 250 cm ‒ Diffuser driving cycle:Aerification worker degree 75 min (50% of main machine 88%+ accessory) stop process 75 Min ‒ Height of dirt field side at stop process:65cm from surface of the water 496 Appendix 7Example of oxidation ditch process model development Tracer experiment when diffuser stops Immediately after the stop process beginning or 10 The tracer was sent to the inflow part of influent and the sending back dirt after min (The lithium chloride; It is 5 as the average concentration in a reactive tank a large amount of mgLi/L), the mixture was collected in the direction of depth in 4-5 places in the tank by three points (70 from the surface and the surface of the water the cm and 150 cm) afterwards, and the lithium concentration in the filtrate was measured. The operating condition when this investigation is executed is as follows. ‒ Influent quantity: 878 M3/d ‒ Sending back dirt flow rate: 1157 M3/ d (1.3 compared with sending back) ‒ Depth of reactive tank: 250 cm ‒ Diffuser driving cycle:Aerification worker degree 75 min (50% of main machine 88%+ accessory) stop process 75 Min ‒ Height of dirt field side at stop process:65cm from surface of the water (3)Measurement result and analysis Measuring water quality after diffuser stops [Mizu] ..adoption.. partFigure A7. 14The change in NH4-N and the NO3-N density in each part after the stop process begins of [ni]Table A7. 5[Ni] was brought together. ・ The surface and 150 in depth The difference is obviously seen in the water quality, and the concentration difference is growing by the cm(Hereafter, it is called, "Dirt layer") with the time passage. ・ The difference of the water quality comes to be shown in the direction of the length of the waterway with the time passage though the difference of the water quality is not seen in three places immediately after the stop process beginning. ・ In modeling the stop process, it can be judged that it is necessary to handle the top layer and the dirt layer independently. 497 Appendix 7Example of oxidation ditch process model development [Mizu] ..adoption.. part Influent and sending back dirt ③ ① ② Final sedimentation tank Figure A7. 14[Mizu] ..adoption.. part after diffuser stops in measuring water quality Table A7. 5Measuring water quality result after diffuser stops Measuremen t part ① ② ③ NH4-N [mg/L] NO3-N [mg/L] Elapsed time min after stop process begins Elapsed time min after stop process begins 0 20 35 50 63 0 20 35 50 63 150cm <0.2 - <0.2 <0.2 1.1 0.3 1.0 0.6 1.4 0.8 3.4 - 2.9 3.3 2.0 2.0 2.3 1.2 2.2 1.1 Surface <0.2 0.2 0.7 0.7 0.8 3.5 3.4 3.1 2.7 2.3 Surface 150cm - <0.2 <0.2 <0.2 <0.2 - 3.1 2.1 1.4 0.9 Surface <0.2 <0.2 <0.2 <0.2 <0.2 3.5 3.3 3.1 2.9 2.8 150cm - <0.2 <0.2 <0.2 <0.2 - 3.0 2.2 1.5 1.3 Tracer experiment when diffuser stops The case and after the stop process begins of sending the tracer, 10 immediately after the stop process beginning Min : the change in [mizu] part and ..adoption.. lithium concentration about each case turned on when passing. Figure A7. 15、Figure A7. 16[Ni] was shown. ・ In the case to have sent the tracer immediately after the stop process beginning, the tracer flowed promptly in part ① near the turning on point and the peak in part ③ is about 10 though the peak of the lithium concentration was not seen at all After min, the peak in part ④ is 60 It is understood that the flow rate in the tank decreased rapidly after the stop process had begun from now on after min it. 498 Appendix 7Example of oxidation ditch process model development However, ④ ..part..60 The peak after min suggests and the style in the tank at that time ..appearing point.. suggests not stopping completely (The backflow need not be considered as shown in the under). ・ Stop process beginning 10 In the case to have sent the tracer after min, the peak in part ② is about 25 After min, the peak in part ③ is about 40 After min. The flow rate in the tank can call a small very much, and proves the result above. Moreover, it can be judged that the backflow of the mixture may be disregarded because the lithium concentration is from beginning to end a low concentration in part ④ and ⑤. Appendix 7Example of oxidation ditch process model development 30 minutes after 60 minutes it turnsafter it onit turns it on 0.00 0.22 0.00 Surface 70cm 150cm 3.8 2.8 0.29 Surface 70cm 150cm 499 30 minutes after 60 minutes it turnsafter it onit turns it on 0.00 0.00 0.22 0.11 0.20 0.15 Influent and sending back dirt ④ ① ③ ② 30 minutes after 60 minutes it turnsafter it onit turns it on 1.8 0.20 1.3 Surface 70cm 150cm < part ① > 0.41 0.18 1.2 Surface 70cm 150cm 5 Lithium concentration mg/L 4 3 tank 30 minutes after 60 minutes it turnsafter it onitFinal turnssedimentation it on 0.80 0.58 1.9 0.50 0.15 0.38 Surface 70cm in depth 150cm in depth 2 1 0 0 < part ③ > 10 20 30 40 50 60 Elapsed time's worth after tracer is turned on 20 Lithium concentration mg/L 15 70 Surface 70cm in depth 150cm in depth 10 5 0 0 < part ④ > 10 20 30 40 50 60 Elapsed time's worth after tracer is turned on 70 5 Lithium concentration mg/L Surface 4 70cm in depth 3 150cm in depth 2 1 0 0 10 20 30 40 50 60 Elapsed time's worth after tracer is turned on 70 Figure A7. 15Tracer test result after diffuser stops (case turned on immediately after stop process beginning) 500 Appendix 7Example of oxidation ditch process model development Presence of backflow Part ① Five minutesTen after minutes it turns30 after it minutes onit turns after 53 it on minutes it turns after it on it turns it Part on Surface 70cm 150cm 1.9 5.7 15 Part ② 0.9 2.5 19 1.1 2.0 4.1 1.3 1.4 1.9 1.9 0.8 0.8 70cm 150cm 2.8 1.6 3.9 1.8 1.1 3.0 150cm Surface 70cm 150cm Five minutesTen after minutes it turns30 after it minutes onit turns after 58 it on minutes it turns after it on it turns it on 0.0 0.0 0.0 70cm 15 minutes after 35 minutes it turns after it55onminutes it turns after it on it turns it Part on Surface ⑤ Surface ④ 0.0 0.0 0.0 0.0 0.2 0.3 0.1 0.2 0.4 15 minutes after 35 minutes it turns after it55onminutes it turns after it on it turns it on 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 Influent and sending back dirt ④ ⑤ ① ③ ② Flowing of direction of direct flow Presence of difference of flowing in direction of depth < part ① > 20 Surface Final sedimentation tank 70cm in depth 150cm in depth [mg/L] 15 10 concentration Lithium 5 0 < part ② > < part ③ > < part ④ > < part ⑤ > 10 60 5 [mg/L] 4 3 concentration Lithium 2 1 0 0 10 6 5 [mg/L] 4 3 concentration Lithium 2 1 0 10 60 5 [mg/L] 4 3 concentration Lithium 2 1 0 0 10 6 5 [mg/L] 4 3 concentration Lithium 2 1 0 0 10 20 30 40 50 60 70 Elapsed time's worth after tracer is turned on 20 30 40 50 60 70 Elapsed time's worth after tracer is turned on 20 30 40 50 60 70 Elapsed time's worth after tracer is turned on 20 30 40 50 60 70 Elapsed time's worth after tracer is turned on 20 30 40 50 60 70 Elapsed time's worth after tracer is turned on Figure A7. 16Tracer test result after diffuser stops (Stop process beginning 10 case turned on Appendix 7Example of oxidation ditch process model development after min) 501 502 Appendix 7Example of oxidation ditch process model development (4)Discussion about modeling of stop process It was 60 though the flow rate in the tank promptly decreased to the stop process than the above-mentioned investigation after the stop process began of the point of causing the difference in the surface part and dirt in the water quality The flow's completely stopping at the process time of about min it clarified that the point and the backflow that not was were able to be disregarded. As a simple model by whom these findings are reflected Appendix 7Example of oxidation ditch process model development 503 Table A7. 6Shown what of [ni] was constructed. The point of modeling is as follows. ・ The flow rate in the direction of the length of the waterway decreases because of the isokinetic after the diffuser stops until it reaches the threshold (flow rate in the lowest tank). ・ When the flow rate in the tank decreases up to the threshold (subsidence beginning flow rate), the [souretsu] model's each division is divided into the supernatant fluid part and dirt by the sludge-liquid interface. ・ The sludge-liquid interface moves in the direction of depth by the isokinetic until it reaches the critical depth (height that can subside). ・ The flow rate in the direction of the length of the waterway of the supernatant fluid part and dirt is assumed to be identical. ・ The whole quantity instantaneously moves to dirt in a floating element, and the biological response progresses only in dirt. ・ A dissolubility element moves according to the flow rate. Diffusion in the sludge-liquid interface is not considered. ・ When the stop process ends and the flow rate in the tank exceeds the subsidence beginning flow rate, the sludge-liquid interface disappears, and becomes a complete mixing condition again in the direction of depth. 504 Appendix 7Example of oxidation ditch process model development Table A7. 6Outline of modeling diffuser stop process < immediately after diffuser stop > One division division ・ It is complete mixing in the direction of depth. ・ It flows by the flow rate immediately before the stop. Surface of the water Bottom < formation and subsidence of sludge-liquid interface > Supernatant fluid part Sludge-liquid interface Dirt ・ Subsidence beginning when flow rate in tank becomes it as follows "Subsidence beginning flow rate" (parameter). ・ The supernatant fluid part and dirt are divided into the direction of depth in the sludge-liquid interface. ・ The sludge-liquid interface moves in the direction of depth by "Sedimentation rate" (parameter)(The isokinetic subsidence is assumed). ・ The subsidence when the height of dirt reaches "Height that can subside" (parameter) stops. ・ The sludge-liquid interface when the flow rate in the tank exceeds the subsidence beginning flow rate by operating the diffuser is canceled. ・ A floating element has the whole quantity in dirt (The biological response is caused only in dirt). ・ Only the movement (Diffusion is not considered) from dirt according to the subsidence to the supernatant fluid part : a dissolubility element. < mass transfer of flow direction > ・ The flow rate in the tank decreases to "Flow rate in the lowest tank" (parameter) because of the isokinetic after the diffuser stops. The flow rate decrease speed is given by "Decay time" (parameter). ・ The supernatant fluid part is assumed to be equal to the flow rate of dirt. ・ All elements are transported by the flow rate in the tank. ・ Transportation in the flow and the opposite direction is not considered. <It flows in OD and go out > ・ To become equal in dirt, the flow rate in the tank moves the volume of water from the inflow part to the other side with the supernatant fluid part though the inflow part of sewage and the sending back dirt (depth) is set on the basis of actual facilities arrangement. ・ As for a floating element, the whole quantity flows in dirt. It doesn't flow out. ・ A dissolubility element is transported according to the movement of water. Appendix 7Example of oxidation ditch process model development OD reactor [Heno] inflow part OD reactor [Karano] outflow part Q DN ,S Q IN+Re ,S Q UP ,S Q IN+Re -Q UP ,S (Figure flows in dirt, and : from dirt at the outflow. ) Q OUT 505 506 Appendix 7Example of oxidation ditch process model development Bibliography 7A) Japan Sewage Works Association: Drainage Japan Sewage Works Association and test method -1997 edition -1997 7B) Alex,J., Tschepetzki,R., Jumar,U., Obenaus,F. and Rosenwinkel,K.-H.: Analysis and design of suitable model structures for activated sludge tanks with circulating flow. Wat.Sci.Tech., Vol.39, No.4, pp.55-60, 1999. 7C) Cinar,.O, Daigger,G.T. and Graef,S.P.: Evaluation of IAWQ Activated Sludge Model No.2 using steady-state data from four full-scale wastewater treatment plants. Wat.Environ.Res., Vol.70, pp.1216-1224, 1998. 7D) Daigger,G. and Nolasco,D.: Evaluation and design of full-scale wastewater treatment plants using biological process models. Wat.Sci.Tech., Vol.31, No.2, pp.245-255, 1995. 7E) Fiter,M., Colprim,J., Poch,M. and Rodriguez-Roda,I.: Enhancing biological nitrogen removal in a small wastewater treatment plant by regulating the air supply. Wat.Sci.Tech., Vol.48, No.11-12, pp.445-452, 2003. 7F) Ghermandi,A., Bixio,D., Thoeye,C. and De Gueldre,G.: Technical-economical evaluation of the operation of oxidation ditches. Wat.Sci.Tech., Vol.52, No.12, pp.133-139, 2005. 7G) Insel,G., Russell,D., Beck,B. and Vanrolleghem,P.A.: Evaluation of nutrient removal performance for an orbal plant using the ASM2d model. Proceedings of Water Environment Federation 76th Annual Technical Exhibition & Conference (CD-ROM), Los Angeles, Oct.11-15, 2003. 7H) M?kinia,J. and Wells,S.A.: A general model of the activated sludge reactor with dispersive flow- I. Model development and parameter estimation. Wat.Res., Vol.34, pp. 3987-3996, 2000. 7I) Meijer,S.C.F., van der Spoel,H., Susanti,S., Heijnen,J.J. and van Loosdrecht,M.C.M.: Error diagnostics and data reconciliation for activated sludge modelling using mass balances. Wat.Sci.Tech., Vol.45, No.6, pp.145-156, 2002. 7J) Stamou,A., Katsiri,A., Mantziaras,I., Boshnakov,K., Koumanova,B. and Stoyanov,S.: Modelling of an alternating oxidation ditch system. Wat.Sci.Tech., Vol.39, No.4, pp.169-176, 1999. 7K) van Veldhuizen,H.M., van Loosdrecht,M.C.M. and Brandse,F.A.: Model based evaluation of plant improvement strategies for biological nutrient removal. Wat.Sci.Tech., Vol.39, No.4, pp.45-53, 1999. Appendix 8Trouble shooting during charibration stepError! Reference source not found. Appendix8Troubleshooting in calibration Here, the matter and each counter measure that should be checked when processing status of the object facilities cannot be reproduced by the simulation at the calibration of the model is illustrated. Also because the majority are the matters that should be confirmed as assumption of work there, it is desirable to confirm these before the adjustment of parameter value though the situation in which the fitting cannot be basically done to the processing results data in "Adjustment of 4.8.8 parameter values etc." is assumed. General point to be checked Whether the following information is appropriately set is confirmed before all the adjustments. Facilities composition (reactive tank division, various ducts, and final sedimentation tanks) Capacity of reactive tank Influent quantity Influent quality Sending back dirt flow rate Internal circulation flow rate Excess sludge flow rate (Or, SRT). Blast volume (Or, KLa and DO control value, etc.). Water temperature Reactive tank initial concentration Numerical calculation method (*t is included). 507 508 Appendix 8Trouble shooting during charibration step Calibration of amount of solid Problem Neither the abundance of the solid nor the reactive tank solid concentratio n are expressible. Check matter Confirmation of handling of amount of solid that flows out as treated water – Is not numerical values with different presence of the amount of the treated water solid compared? Confirmation of measurement data integrity of amount of generation solid Counter measure It compares it by the abundance of the solid including the amount of the outflow solid as the treated water (Because the treated water solid concentration is expected according to the final sedimentation pond model, this is treated easily). At least, data where Rin's revenue and expenditure is taken is used. The average data of half month the frequency of the data that can be used and after the constancy of processing status is considered about one month is used to ease the influence of the difference of short-term data. The changed portion is corrected when the results data is hit at the change time of the amount of the solid in the faction and the amount of the generation solid is calculated. An additional investigation to understand the abundance of the solid accurately is done. – Is Rin's revenue and expenditure taken? – Do not you calculate by data with scarce representative character for a short term? – Isn't the reactive tank solid concentration in increase/decreasing tendency at the results data collection time? Confirmation of SRT – Is SRT in the simulation corresponding of the results value? The excess sludge flow rate etc. are adjusted so that SRT may agree with the results value (Being able to trust the results value of SRT is assumption). Appendix 8Trouble shooting during charibration stepError! Reference source not found. Confirmation of validity of solid index and conversion factor – When MLSS and MLVSS are assumed to be an index, is the conversion method from the element of the model appropriate (Are neither XTSS, MLSS nor MLVSS simply compared?)? – Similarly, is conversion factor to XTSS appropriate (comparison etc. between the actual measurement value and calculated value of the XTSS /CODCr ratio of dirt)? Indices that can be compared are compared. It is discussed to make CODCr an index to exclude the uncertainty concerning the conversion factor. To be expressible of the actual measurement value of the XTSS /CODCr ratio when MLSS and MLVSS are assumed to be an index, conversion factor (iTSS, XI, iTSS, and BM, etc.) is adjusted. Confirmation of influent quality – Has not the segmentation resulted high (>30%) as the abundance ratio of XI is extreme? The XI /XS ratio of influent is adjusted. 509 510 Appendix 8Trouble shooting during charibration step Calibration of nitrification Problem Check matter Counter measure The NH4-N Confirmation of SRT density of a – Is SRT in the simulation conceited corresponding of the results value? tank and the treated water is not expressible. The excess sludge flow rate etc. are adjusted so that SRT may agree with the results value (Being able to trust the results value of SRT is assumption). Confirmation of DO density – Can DO of the conceited tank each division reproduce the actual measurement value? The scatter nature model is adjusted so that the DO density may become the measurement value equivalent (KLa, blast volume, oxygen solubility efficiency, and DO control value, etc.). The DO concentration profile of a conceited tank is investigated (You may investigate the DO concentration distribution in the tank handled as one division on the model together). The tank division on the model is changed according to the result. DO in the simulation is compulsorily changed when the measurement data of DO is all unavailable and behavior is confirmed. Confirmation of nitrogen balance – Is the revenue and expenditure elements of the amount of the inflow nitrogen and the amount etc. of the excess sludge nitrogen corresponding of the results value? Confirmation of XAUT density of influent – Is not both initial values of XAUT of influent and XAUT of a reactive tank set to 0? – Isn't the XAUT density of influent excessive compared with the amount in the faction? IN and Xs, etc. are adjusted so that inflow T-N density may become the results value equivalent. IN, BM, and iNXI, etc. are adjusted so that the amount of the excess sludge nitrogen may become the results value equivalent (It is assumption appropriately expressible of the amount of the excess sludge solid). The density of influent XAUT of extent that doesn't influence the amount in the faction is set. Appendix 8Trouble shooting during charibration stepError! Reference source not found. Confirmation of expression of NH4-N density of reluctance/hypoxia tank – Is SNH4 of the uppermost in reactive tank style division expressible? To be expressible of the NH4-N density of the sending back dirt, the final sedimentation pond model is adjusted. The validity of the sending back dirt flow rate set point is confirmed by the MLSS revenue and expenditure in the tank circumference. The blast volume (amount of the oxygen supply) is adjusted for the pseudoreluctance tank. ..nitrification.. ..calibration (.. ..continuation..) Problem The NH4-N density of a conceited tank and the treated water is not expressible. ..(.. ..continu ation..) Check matter Counter measure Confirmation of limiting by PO4-P – Is not the limiting by SPO4 caused in the proliferation process of XAUT in the simulation (The value of the rate equation of clause SPO4 is confirmed)? K P value according to XAUT is reduced, and the limiting by SPO4 is eased. Confirmation of limiting by alkalinity – Is not the limiting by SALK caused in the proliferation process of XAUT in the simulation in facilities of the reality though the pH has not decreased (The value of clause SALK of the rate equation is confirmed)? The SALK density set point of influent is reviewed. The KALK value according to XAUT is reduced, and the limiting by SPO4 is eased. Calibration of denitrification Problem The NO3-N density of the hypoxia tank is not expressible. Check matter Confirmation of circulation liquid – DO Counter measure density of Is the DO density of a conceited tank corresponding of the results value? Refer to "Calibration of nitrification". 511 512 Appendix 8Trouble shooting during charibration step Confirmation of mixture situation of reactive tank – Is not the backmixing caused before and behind the hypoxia tank in the object facilities? – Is not the inclination of the NO3-N density etc. made to one division? The NO3-N Confirmation of simulation of density of a nitrification conceited – Is the SNH4 profile of a conceited tank and the tank expressible? treated water is not Confirmation of denitrification expressible. situation in conceited tank – Do not it excessive/underestimate the amount of the denitrification in a conceited tank? This is reflected in the simulation when there is a possibility of the backmixing. The tank number of partitions of the hypoxia tank is changed. Refer to "Calibration of nitrification". KO2 according to XH is adjusted, and it ..amount of the denitrification in a conceited tank.. corresponds in the results value. When the DO density of a conceited tank is unclear, the DO density along with the calibration of nitrification is adjusted. Appendix 8Trouble shooting during charibration stepError! Reference source not found. Calibration of phosphorus removal Problem Check matter Counter measure The PO4-P Confirmation of presence of PAO density of – In the results data, are Rin's the discharge and uptake seen? reluctance tank is not expressible. When the behavior of PO4-P by PAO is not seen, the calibration according to the biological phosphorus removal is difficult. Confirmation of NO3-N inlet flow of sending back dirt origin – Is the NO3-N density of the sending back dirt corresponding of the results value? To be expressible of the NO3-N density of the sending back dirt, the final sedimentation pond model is adjusted. Confirmation of DO inlet flow of influent and sending back dirt origin – Does the DO density set point of influent reflect results? – Does the DO density set point of the sending back dirt reflect results? Confirmation of mixture situation of reactive tank – Is not the backmixing caused between reactive tanks in the downstream? – Is the tank division of the reluctance tank appropriate? Amount of oxygen supply in pseudoreluctance tank and confirmation of stir situation SO2 of influent is assumed to be results equivalent (The data collection of the addition is done if necessary). To be expressible of the DO density of the sending back dirt, the final sedimentation pond model is adjusted. This is reflected in the simulation when there is a possibility of the backmixing. The tank number of partitions of the reluctance tank is changed. Does the blast volume in the pseudoreluctance tank appropriately reflect results? – The stir in the pseudoreluctance tank is enough. The blast volume (amount of the oxygen supply) is adjusted. The tank number of partitions is changed. The division of the small capacity which influent and the sending back dirt flow in is installed. The PO4-P Confirmation of mixture situation of density of a reactive tank conceited – Is not the backmixing caused tank and the between reluctance/hypoxia tank? treated water – Is not the backmixing caused in the is not division of a conceited tank? expressible. This is reflected in the simulation when there is a possibility of the backmixing. – 513 514 Appendix 8Trouble shooting during charibration step Confirmation of limiting by NH4-N – Isn't the SNH4 density in the simulation remarkable and limiting [shi] as for proliferation and the XPP store process of XPAO? The KNH4 value according to XPAO is reduced, and the limiting by SNH4 is eased. 515 Address of thanks The content of this report is based on the result of investigation "Development of the activated sludge model (advanced technology development project)" (1999-2001), "Design using the activated sludge model and development of the maintenance technique" (2001-2003), and "Design using the activated sludge model and investigation concerning the applicability of the maintenance technique" (2004-2005) that the JS technology development part executed. We wish to express our thick gratitude to the parties concerned all of you in the sewage plant in which it cooperated in the data collection and hearing in the process of these investigations. Moreover, describe the use of the result of joint research "Verification ..application..- the design and the driving management support of development-oxidation ditch method of a business approach of the activated sludge model" with JS and JFE engineering Ltd. that executes it clearly in 2001-2004, and in "Chapter 5 case study" and "Discussion case with the process model of seven appendix oxidation ditch methods" among this reports, we wish to express our gratitude to the parties concerned all of you of this company.