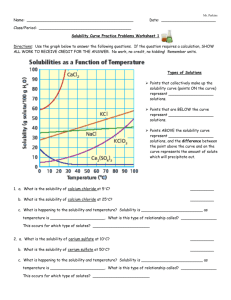
Solubility Curve Practice Problems Worksheet 1
advertisement

Solubility Curve Practice Problems Worksheet Name________________________ Directions: Use the graph below to answer the following questions. If the question requires a calculation, SHOW ALL WORK TO RECEIVE CREDIT FOR THE ANSWER. No work, no credit, no kidding! Remember units. Types of Solutions Points that collectively make up the solubility curve (points ON the curve) represent ___________________ solutions. Points that are BELOW the curve represent ___________________ solutions. Points ABOVE the solubility curve represent ___________________ solutions, and the difference between the point above the curve and on the curve represents the amount of solute which will precipitate out. 1. a. What is the solubility of calcium chloride at 5C? b. What is the solubility of calcium chloride at 25C? ___g/100g of H2O ___g/100g of H2O c. What is happening to the solubility and temperature? Solubility is ___Increasing or Decreasing___ as temperature is __Increasing or Decreasing___. What is this type of relationship called? __direct or indirect___. This occurs for which type of solutes? ___gasses or solids____ 2. a. What is the solubility of cerium sulfate at 10C? ___g/100g of H2O b. What is the solubility of cerium sulfate at 50C? ___g/100g of H2O c. What is happening to the solubility and temperature? Solubility is ___increasing or decreasing____ as temperature is ___increasing or decreasing_____. What is this type of relationship called? __direct or indirect__ 3. a. At 90C, 10 g of potassium chlorate is dissolved in 100. g of water. Is this solution saturated, unsaturated, or supersaturated? b. How do you know? Solubility Curve Practice Problems Worksheet Name________________________ 4. A saturated solution of potassium chlorate is dissolved in 100. g of water. If the saturated solution is cooled from 90C to 60C, how many grams of precipitate will be formed? __________ 5. Which substance on the graph is least soluble at 10C? __________ 6. Which substance on the graph shows the least change in solubility from 0C to 100C? __________ Complete the following equations when the following ionic solids get placed in water naming the ions and the compounds: Example: Ca(OH)2 ---> Answers: Ca(OH)2 ---> Calcium Hydroxide 7. K2CO3 ---> 8. Na2CO3 ---> 9. NaC2H3O2 ---> 10. Al2(SO4)3 ---> 11. FeBr2 ---> 12. CuCl2 ---> 13. Pb(NO3)2 ---> 14. CuS ---> 15. MgSO4 ---> 16. Pb(NO3)2 ---> 17. FeCl2 ---> 18. FeBr3 ---> Ca+2 + 2OH- Calcium Ion + Hydroxide